Question
Question: Zone-refining has been employed for preparing ultra-pure samples of: A: \( Cu \) B: \( \;Zn \) ...
Zone-refining has been employed for preparing ultra-pure samples of:
A: Cu
B: Zn
C: Ge
D: Ag
Solution
Hint : The process or method of purifying an impure metal is known as refining of metal. After extraction from the raw materials, crude metals comprise 96-99% principal metal and rest is impurity so it is important to undergo metal refining as crude metals have inferior physical, chemical or mechanical properties and cannot be used by industries.
Complete Step By Step Answer:
Method of Zone refining is also known as zone melting. This method is generally used for refining of the metals possessing a low melting point. In this technique, a circular form of mobile heater is usually fixed at one corner of a rod made from impure metal. In this method, the impurities are mainly concentrated at one end of the block of metal such that the rest of the block gets purified. This technique is utilised in the case where impurities dissolve in molten state in comparison to that in solid form of the metal. This process is very effective for the removal of impurities from the semiconducting elements silicon, gallium, germanium.
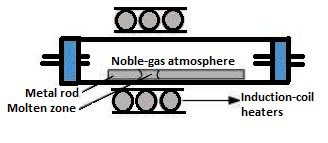
Among the given options, Cu , Zn and Ag are purified through electrolytic refining methods. While Ge is purified Zn is purified through a zone refining method.
Hence, the correct answer is Option C.
Note :
Refining process differs from other processes like calcining or smelting which generally include a chemical change in the raw material. On the other hand, in the refining process, the final product is chemically identical to the raw material i.e. original one (only it is its purest form).
