Question
Question: Zn(OH)₂ is an amphoteric hydroxide and is involved in the following two equilibria in aqueous soluti...
Zn(OH)₂ is an amphoteric hydroxide and is involved in the following two equilibria in aqueous solutions?
Zn(OH)2⇌Zn2+(aq)+2OH−(aq);Ksp=1.2×10−17
Zn(OH)2(s)+2OH−(aq)⇌[Zn(OH)4]2−(aq);Kc=0.12 At what pH the solubility of Zn(OH)2 be minimum?
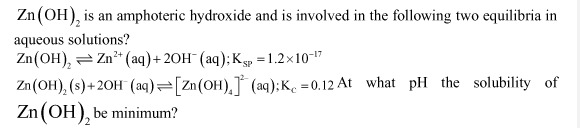
10
Solution
The solubility of Zn(OH)2 in aqueous solution is determined by two equilibria:
-
Dissolution to form simple ions:
Zn(OH)2(s)⇌Zn2+(aq)+2OH−(aq);Ksp=[Zn2+][OH−]2=1.2×10−17
-
Dissolution to form a complex anion in the presence of excess hydroxide:
Zn(OH)2(s)+2OH−(aq)⇌[Zn(OH)4]2−(aq);Kc=[OH−]2[[Zn(OH)4]2−]=0.12
The total solubility, S, of Zn(OH)2 is the sum of the concentrations of all zinc-containing species in solution, excluding the solid Zn(OH)2. In this case, these are Zn2+ and [Zn(OH)4]2−.
S=[Zn2+]+[[Zn(OH)4]2−]
From the first equilibrium, we can express [Zn2+] in terms of [OH−]:
[Zn2+]=[OH−]2Ksp
From the second equilibrium, we can express [[Zn(OH)4]2−] in terms of [OH−]:
[[Zn(OH)4]2−]=Kc[OH−]2
Substituting these expressions into the equation for total solubility:
S=[OH−]2Ksp+Kc[OH−]2
To find the pH at which the solubility is minimum, we first find the concentration of [OH−] at which the solubility S is minimum. We treat S as a function of [OH−] and find the minimum by taking the derivative with respect to [OH−] and setting it to zero. Let x=[OH−].
S(x)=Kspx−2+Kcx2
Differentiating S(x) with respect to x:
dxdS=−2Kspx−3+2Kcx
Set the derivative to zero to find the critical points:
−2Kspx−3+2Kcx=0
2Kcx=2Kspx−3
Kcx=Kspx−3
Kcx4=Ksp
x4=KcKsp
x=(KcKsp)1/4
Substitute the given values of Ksp and Kc:
Ksp=1.2×10−17
Kc=0.12=1.2×10−1
[OH−]=(1.2×10−11.2×10−17)1/4
[OH−]=(10−16)1/4
[OH−]=10−4M
This is the concentration of hydroxide ions at which the solubility of Zn(OH)2 is minimum.
Now, we convert this concentration to pOH:
pOH=−log10[OH−]
pOH=−log10(10−4)
pOH=4
Finally, we convert pOH to pH using the relationship pH+pOH=14 (at 25∘C):
pH=14−pOH
pH=14−4
pH=10
Thus, the solubility of Zn(OH)2 is minimum at pH 10.
