Question
Question: Zeigler Natta catalyst is: (A) \[Pt/PtO\] (B) \(Al{({C_2}{H_5})_3} + TiC{l_4}\) (C) \(K[PtC{l_...
Zeigler Natta catalyst is:
(A) Pt/PtO
(B) Al(C2H5)3+TiCl4
(C) K[PtCl3(η2−C2H4)]
(D) Pt/Rh
Solution
Catalyst is a substance that increases the rate of a reaction without consuming.
Enzymes are natural catalysts and used in money biochemical reactions.
Zeigler Natta catalyst used in polymerization of ether to from polyether
Step by step answer:
Zeigler Natta catalyst is an organometallic compound.
This is formed by a combination of aluminum trialkyl with the salt of transition metal i.e., Titanium tetrachloride.
Therefore, formula of Ziegler Natta catalyst is
Therefore, from the above explanation the correct option is (B).
Zeigler Natta catalyst is Heterogeneous catalyst.
Heterogeneous means the compound consists of more than one phase. In this catalyst organometallic compound and titanium tetrachloride is present.
This helps in polymerization of olefins to polymers of high molecular weight and highly ordered structure.
Olefins are hydrocarbons containing a double carbon-carbon bond.
There are two types of polythene:
(i) Low density polythene: They are formed by highly branched structures. It is obtained by the polymerization there under high pressure of atm. At to in presence of peroxide.
(ii) High density polythene: They are linear molecules and have high density due to close packing.
It is formed by polymerization of ethane in presence of Ziegler Natta catalyst at temperature to and atmospheric pressure.
Mechanism: High density polymers are formed by chain growth mechanisms.
And chain growth mechanism takes place through free radical mechanisms.
Zeigler Natta catalysts help in coordination polymerization in which complexes are formed between Transition metals and electrons of monomers.
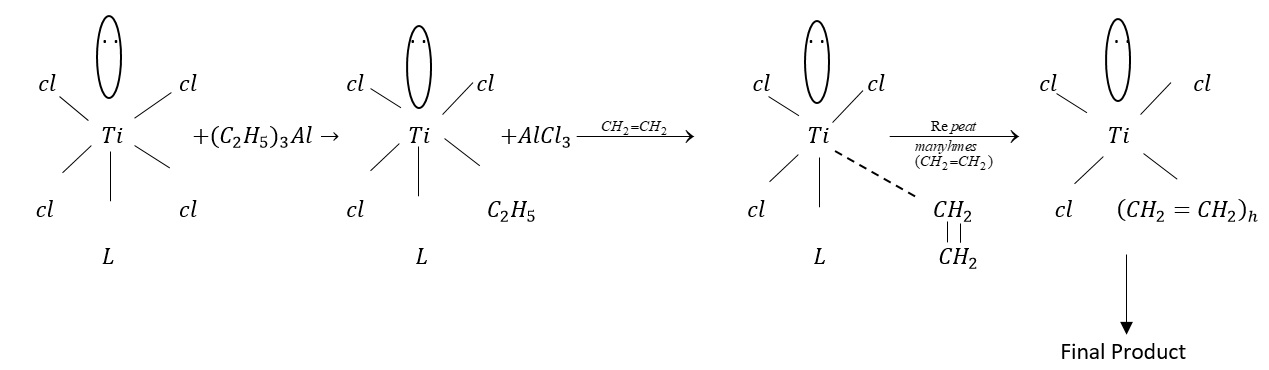
Note: Zeigler Natta catalyst polymerization has some limitations it does not work for some monomers product line polyvinyl chloride cannot be generated by this method.
