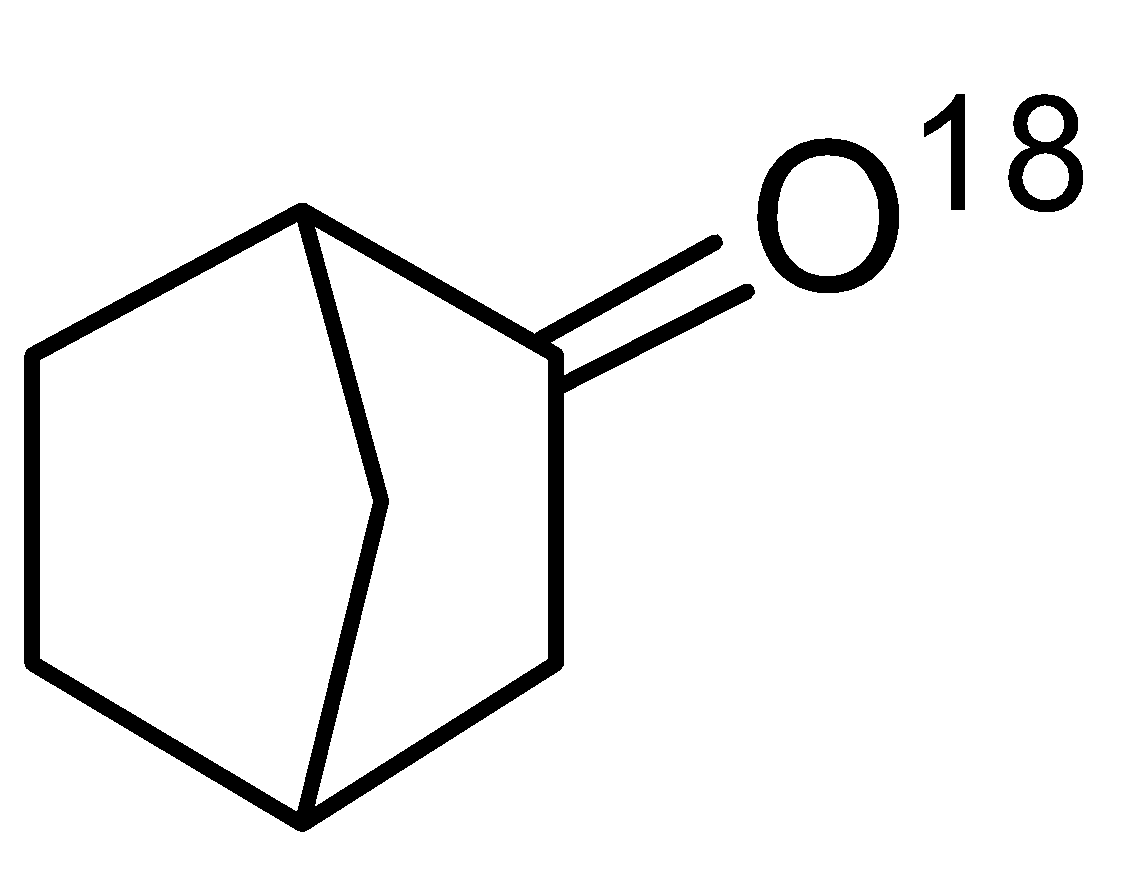
Question
Question: Your OC teacher –Mr. Don decided that the intermediate which forms during the reaction is known as B...
Your OC teacher –Mr. Don decided that the intermediate which forms during the reaction is known as Bahubali. How many Bahubali and Major products?
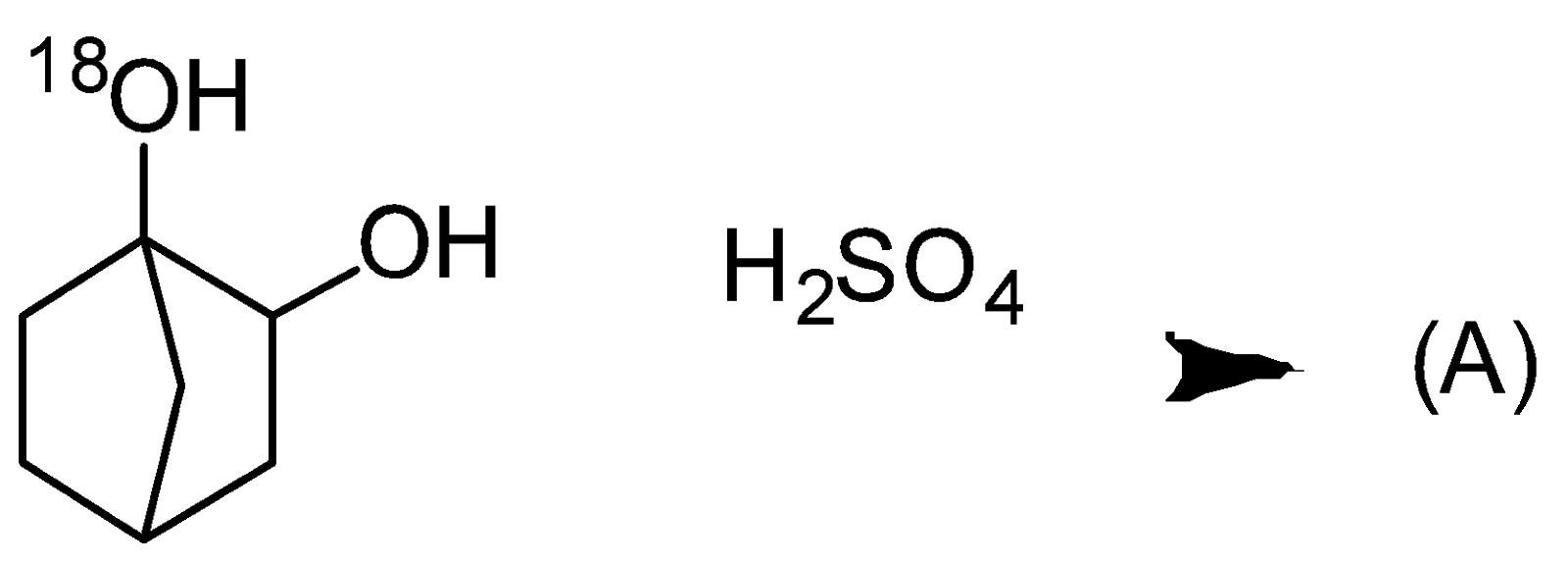
- A. Bahubali is 2
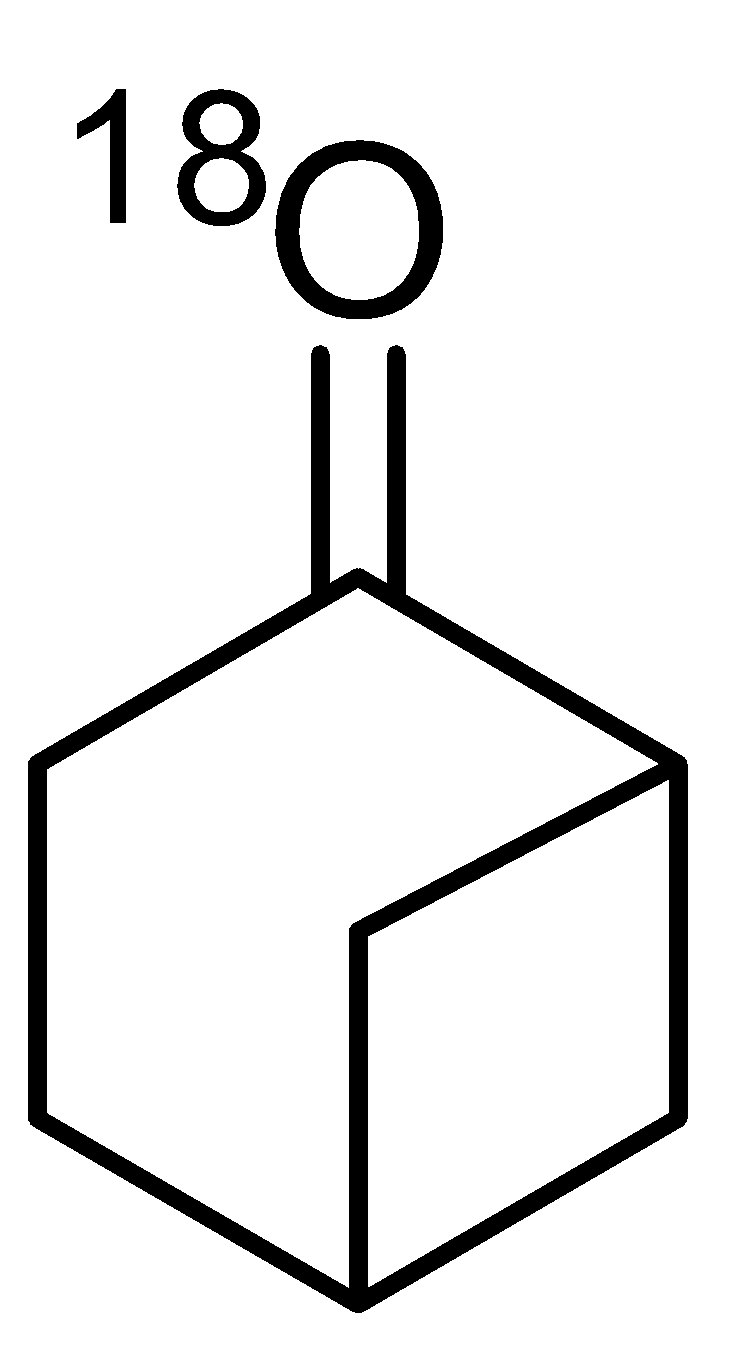
- A. Bahubali is 4
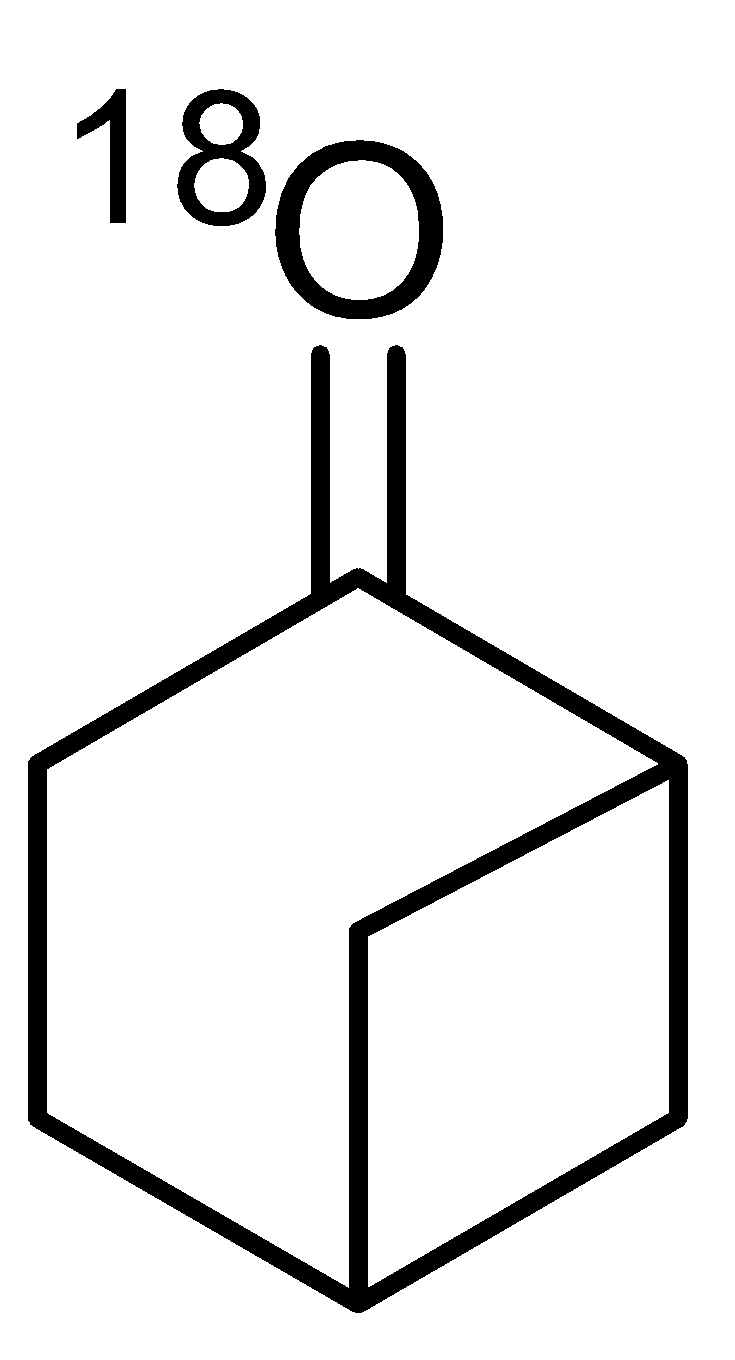
- A. Bahubali is 2
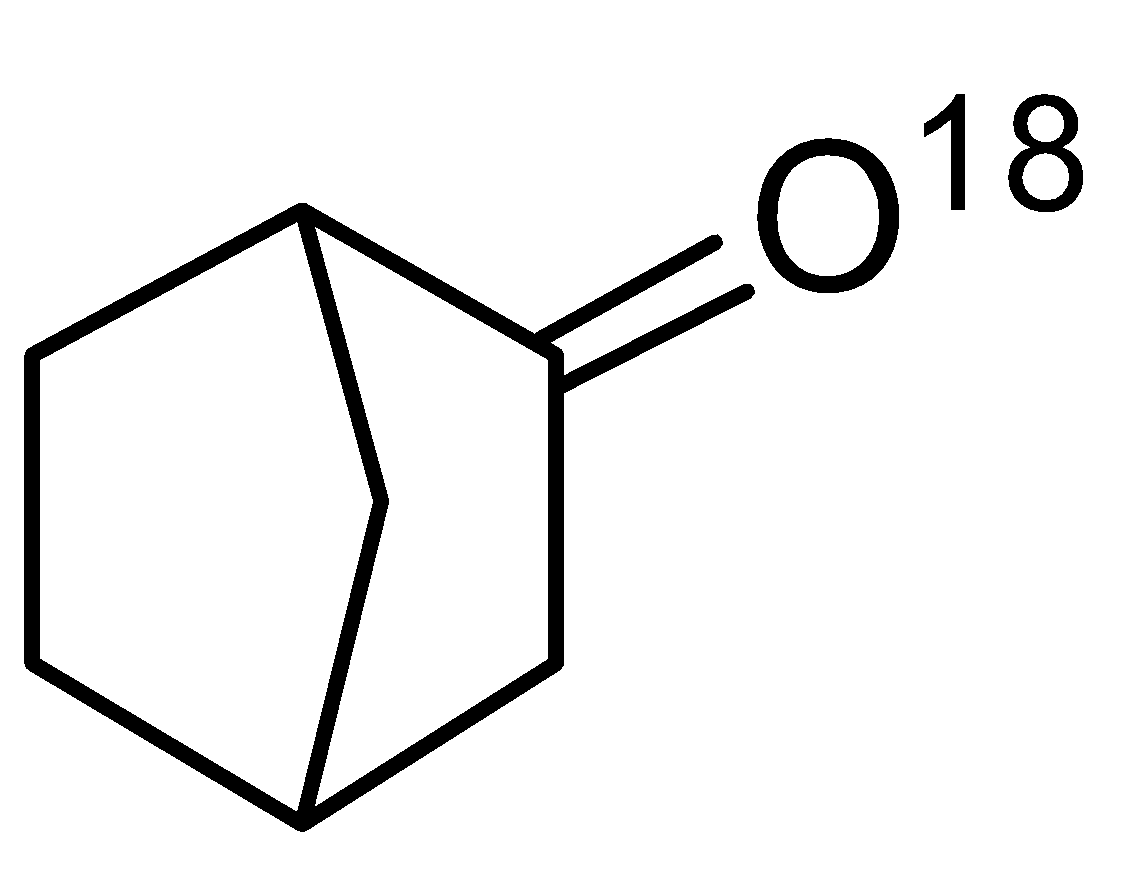
- A. Bahubali is 4
Solution
Hint : In the given reaction, a bridging compound is given which reacts with Sulphuric acids (H2SO4) . We need to find the intermediate and the major product. When alcohol reacts with sulphuric acids (H2SO4) , it leads to an elimination reaction of alcohols.
Complete Step By Step Answer:
When the alcohol reacts with hydrohalic acids such as HCl,HBr and HI , it leads to the formation of alkyl halide from alcohols. Basically, it undergoes a substitution reaction. It proceeds through the SN1 mechanism which includes the protonation of alcohol and a carbocation is formed after the loss of H2O , then the nucleophile attacks the carbocation form.
In the other case, when the alcohol is treated with acids such as H2SO4,H3PO4 and TsOH , it undergoes elimination product which leads to the formation of alkenes. It proceeds through the E1 mechanism.
When alcohol reacted with sulphuric acids (H2SO4) , it includes the formation of carbocation, then the carbocation formed is attacked by the anion of sulphuric acids i.e. −OSO3H and a double bond is formed.
This happens because the anion of sulphuric acids is a very weak nucleophile which is being stabilized by resonance. The negative charge is delocalized over three oxygen atoms. This reason is same in case of anions of TsOH and H3PO4 acids like TsO− and H2PO4− .
The following reaction happen for the given reaction:
Step1- Protonation of alcohol
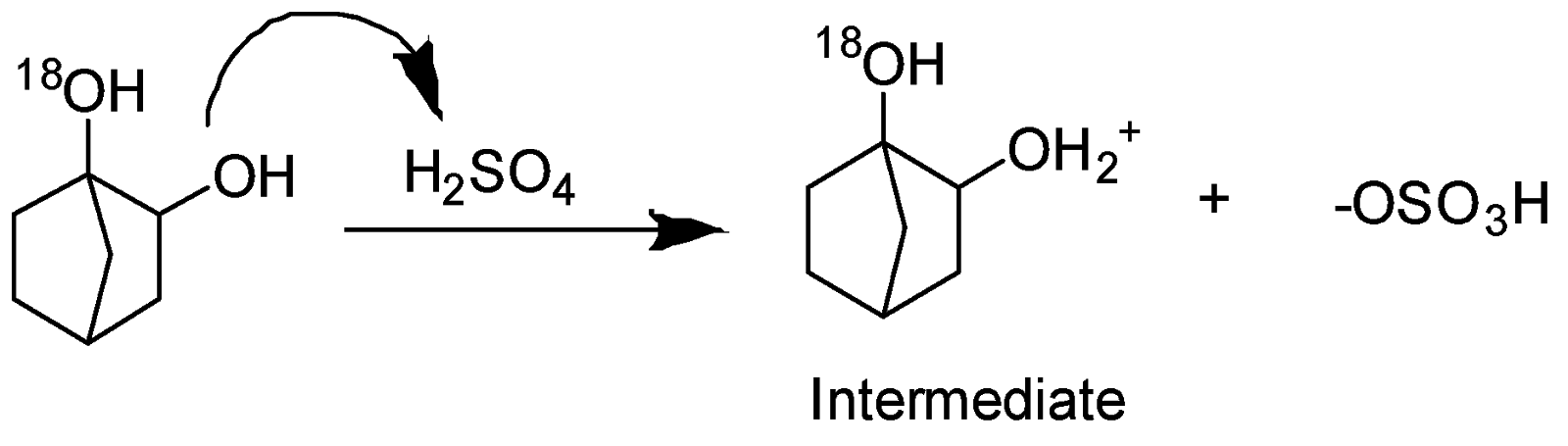
Step -2 Loss of leaving group and formation of carbocation.
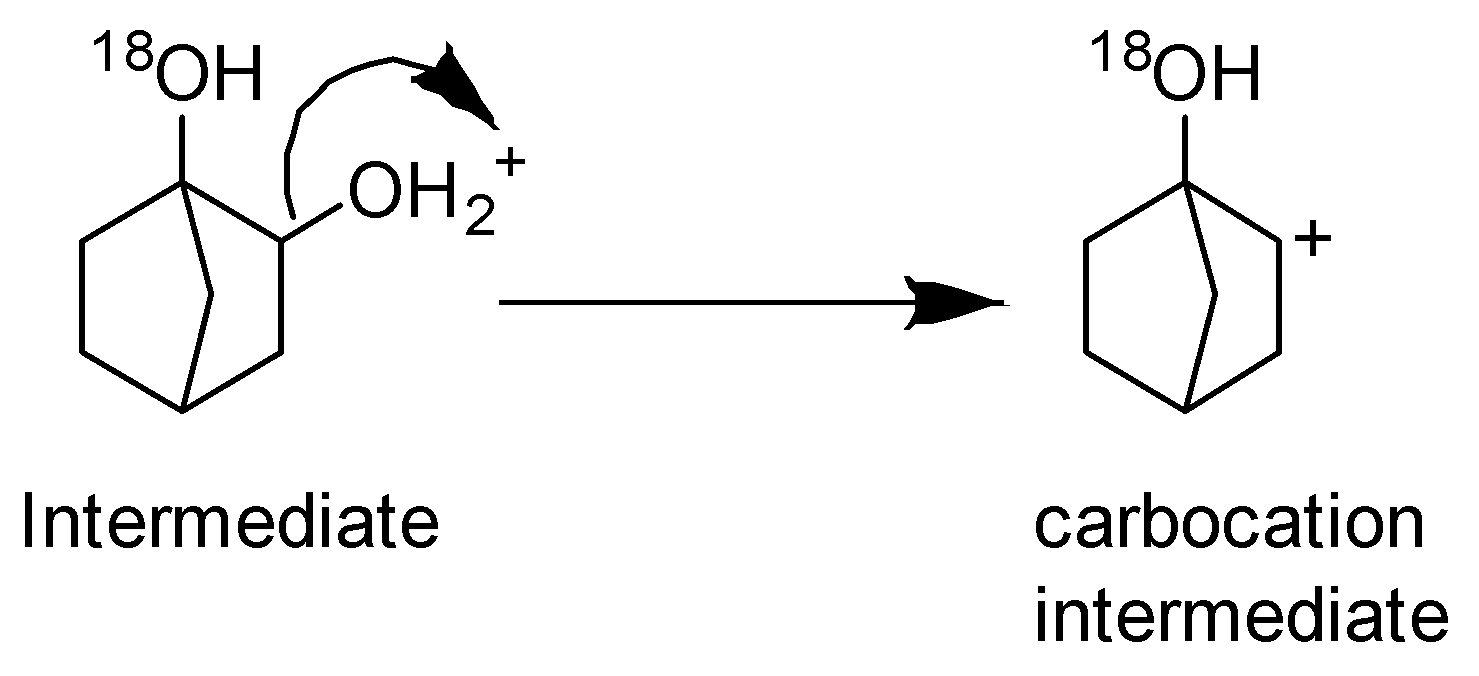
Step-3 Deprotonation to form an alkene.
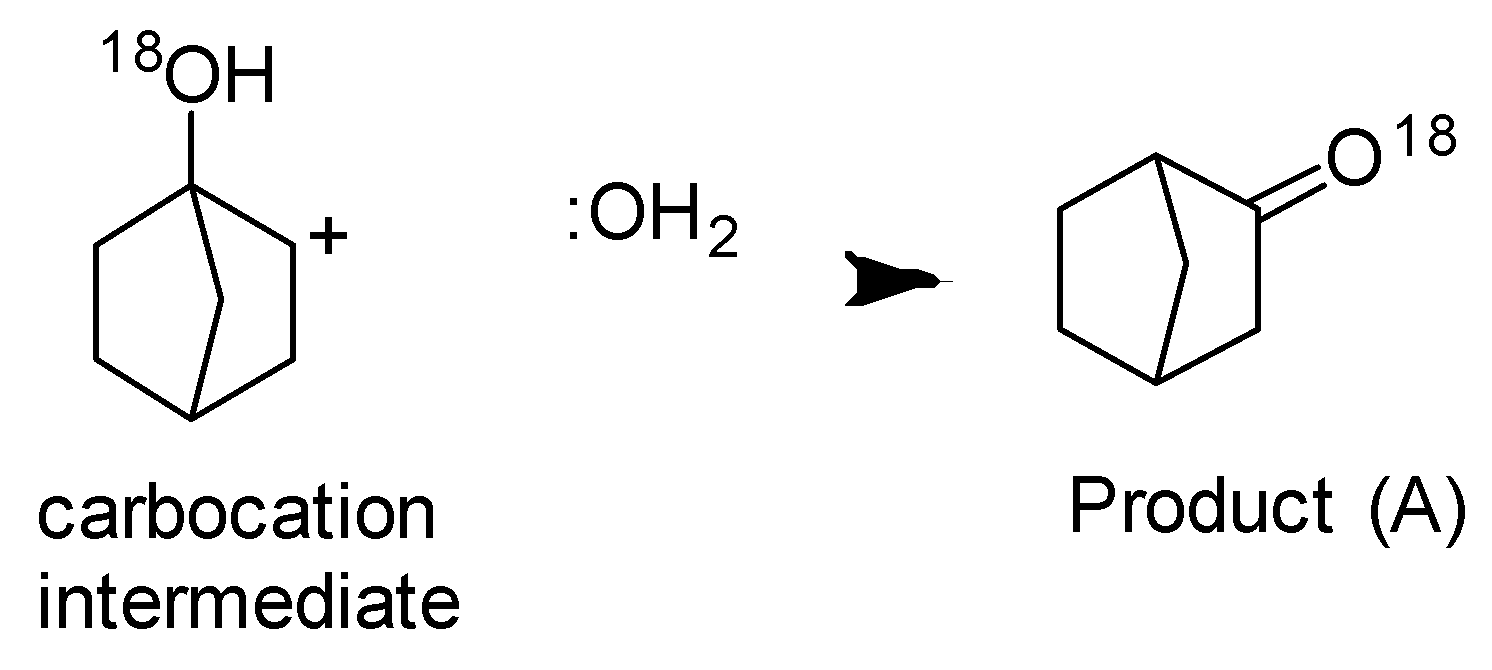
We can see that two intermediates are formed, hence Bahubali is two and the major product will be one.
Hence, the correct answer is option(C).
Note :
It must be remembered that if the secondary alcohol undergoes elimination reaction then the major product is decided by Zaitsev rule. According to this rule, the most substituted alkene is the major product. Trans alkenes will be favoured more as compared to cis alkenes due to steric hinderance.
