Question
Question: You are given benzene, conc. \({{\rm{H}}_{\rm{2}}}{\rm{S}}{{\rm{O}}_{\rm{4}}}\) and \({\rm{NaOH}}\)....
You are given benzene, conc. H2SO4 and NaOH. Write the equations for the preparation of phenol using these reagents.
Solution
In synthesis two or more compounds or elements combine to make a more complex substance. To form benzene sulphonic acid we have to use benzene and conc. sulphuric acid in presence of heat.
Complete step by step answer:
We know that benzene is an organic compound and molecular formula is C6H6. Benzene reacts with concentrated sulphuric acid (conc.H2SO4) in presence of heat and form benzene sulphonic acid. Benzene sulphonic acid is an organosulfur compound with formula C6H6O3S. Now, we write the reaction between benzene and conc. sulphuric acid in presence of heat is as follows:
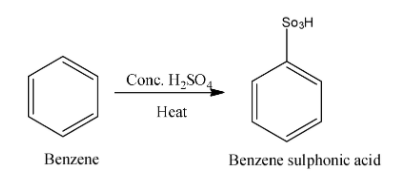
After formation of benzene sulphonic acid it is then heated with sodium hydroxide and form sodium phenoxide. Now we write the reaction between benzene sulphonic acid and sodium hydroxide in presence of heat is as follows:
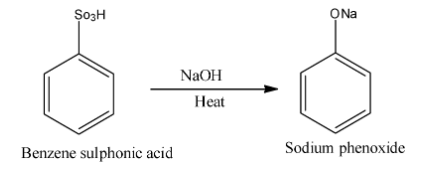
After formation of sodium phenoxide hydrolysis of sulphuric acid given phenol. Sodium phenoxide is an organic compound with molecular formula NaOC6H5. For the formation of phenol from sodium phenoxide heat is not required. We write the reaction of formation of phenol is as follows:
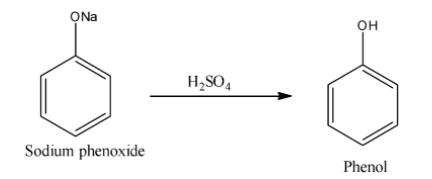
Additional Information: Phenol is a volatile solid that is found in white crystalline form. Phenol contains one −OH group. Phenol is an aromatic compound and molecular formula is C5H6OH. Phenols are used as disinfectants, mouthwash, household cleaners, antiseptics etc.
Note: Hydrolysis is a chemical reaction which one or more chemical bonds break up by a molecule of water. The term is commonly used for replacement, reduction and solving reactions in which nucleophile is water. In industry hydrolysis is commonly used to break down chemicals into smaller fractions or parts. Do not confuse this formation of phenol reaction with sulfonation reaction.
