Question
Question: You are given balls and stick models of six carbon atoms and fourteen hydrogen atoms and a sufficien...
You are given balls and stick models of six carbon atoms and fourteen hydrogen atoms and a sufficient number of sticks. In how many ways one can join the models of six carbon atoms and fourteen hydrogen atoms to form different molecules of C6H14 ?
Solution
Molecules can be represented in a 3D structure using various physical models to understand their structure and orientations. These are called molecular models. Ball and stick model is a molecular model in which balls are used to represent atoms and sticks represent bonds.
Complete answer:
From the hint given, we understood that balls are used to represent atoms and sticks are used to represent bonds in a ball and stick molecular model. The given compound, C6H14 is an alkane with six carbon atoms and fourteen hydrogen atoms. Alkanes are hydrocarbons that have carbon-carbon and carbon-hydrogen single bonds. Their general structural formula is CnH2n+2 . Therefore, the molecule C6H14 will contain nineteen sticks or single bonds.
C6H14 is the molecular formula of hexane and now we have to find its number of possible structural isomers. They are:
1. 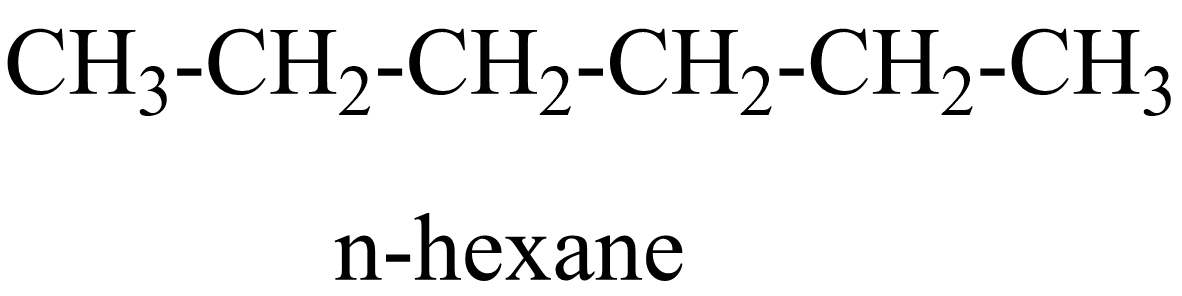
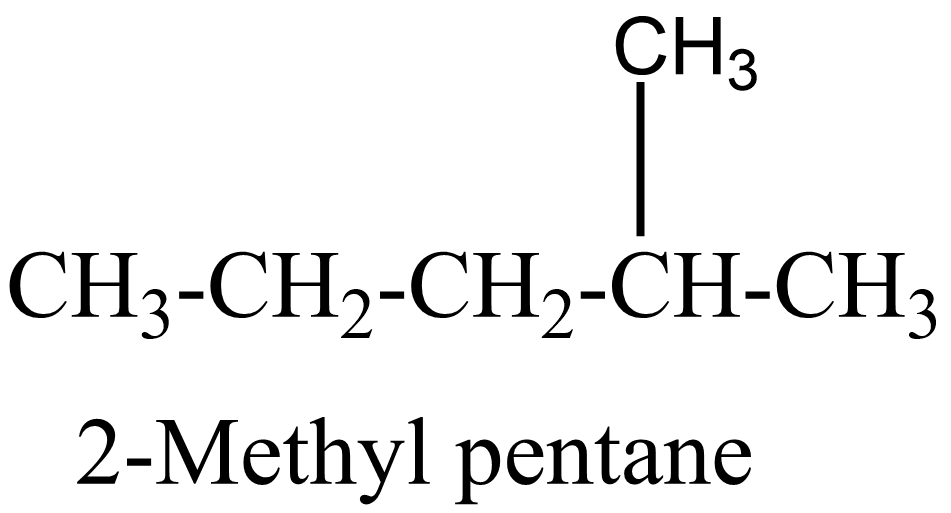
2. 
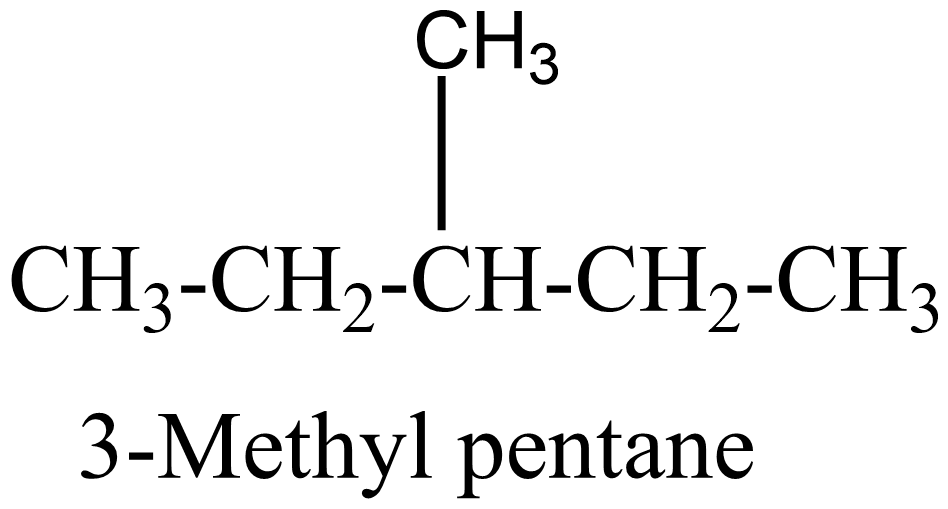
3. 
4 .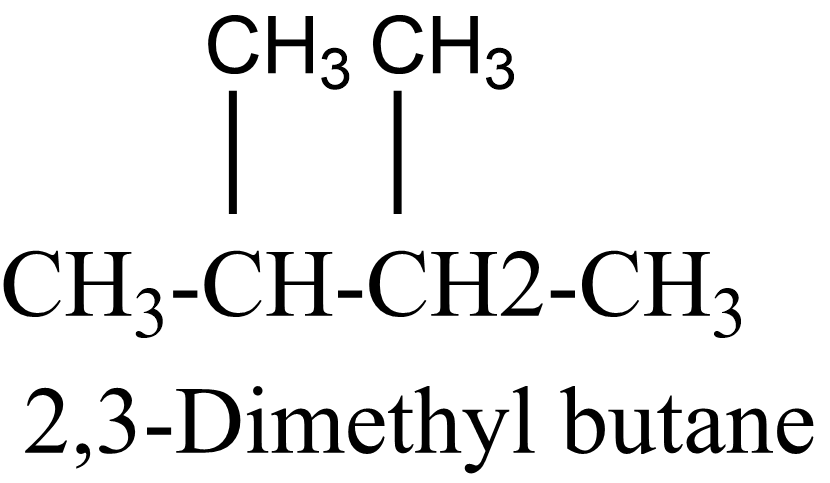
5 . 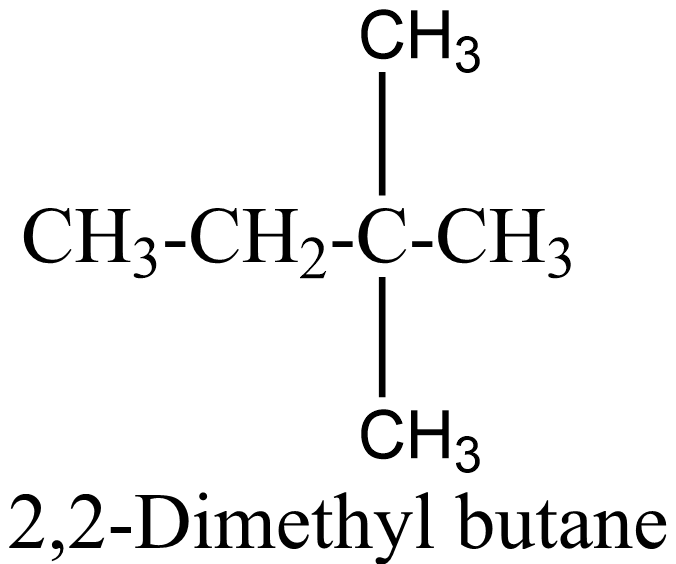
Therefore, C6H14 has 5 structural isomers and thus 5 ball and stick models can be formed from the given number of balls and sticks.
In the above isomers, n-hexane is a straight chain isomer whereas others are branched chain isomers. These isomers have different physical and chemical properties due to the difference in arrangement of atoms.
Note:
Isomers are molecules with the same molecular formula but different structural formulas. They contain the same number of atoms but differ in their arrangement in space. Structural or constitutional isomerism is a form of isomerism in which bonds between the atoms are different. In stereoisomerism, bonds between the atoms are the same but their relative positions are different.
