Question
Question: You are given a one molal solution of each of the following compounds. (A) Glucose, (B) NaCl, (C) ...
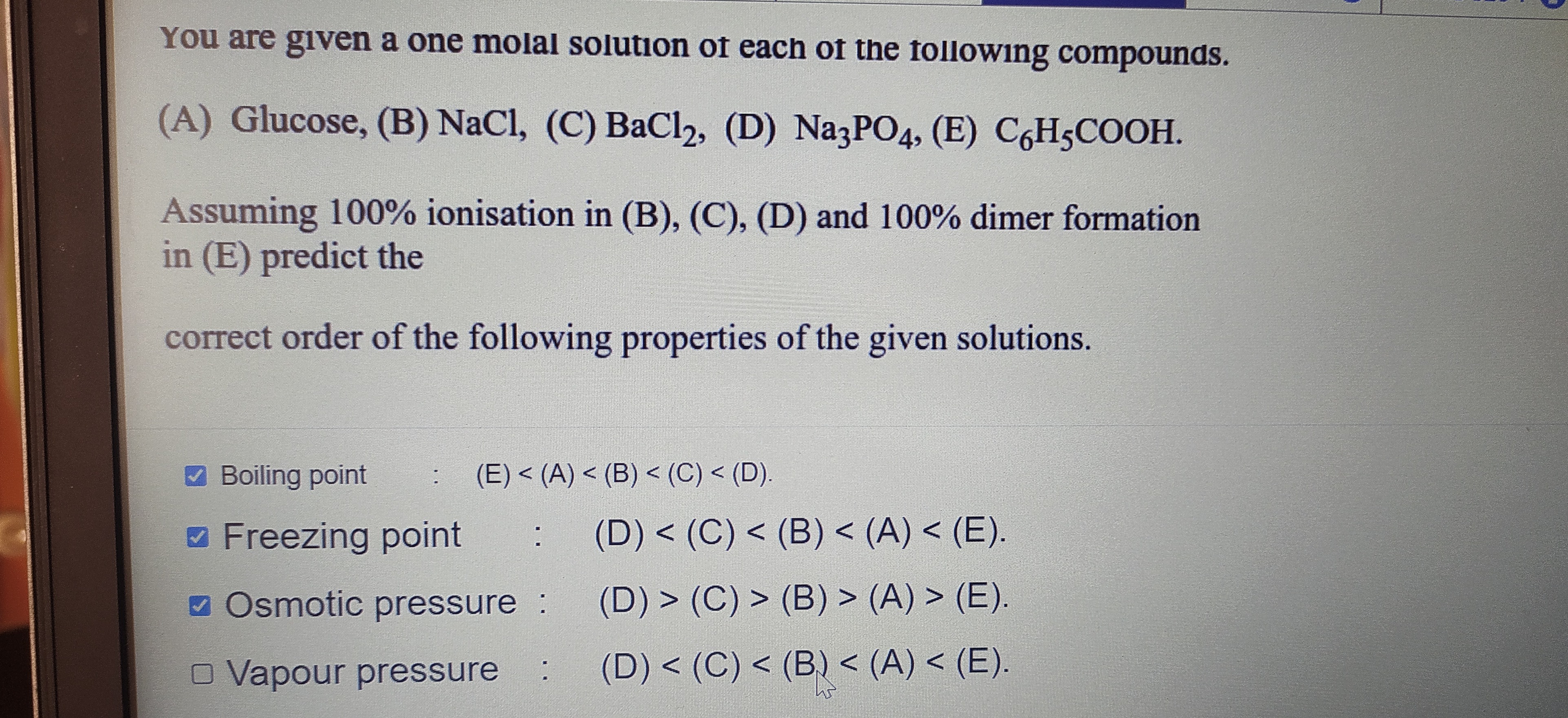
You are given a one molal solution of each of the following compounds.
(A) Glucose, (B) NaCl, (C) BaCl2, (D) Na3PO4, (E) C6H5COOH.
Assuming 100% ionisation in (B), (C), (D) and 100% dimer formation in (E) predict the correct order of the following properties of the given solutions.
- Boiling point : (E) < (A) < (B) < (C) < (D).
- Freezing point : (D) < (C) < (B) < (A) < (Ε).
- Osmotic pressure : (D) > (C) > (B) > (A) > (Ε).
- Vapour pressure : (D) < (C) < (B) < (A) < (E).
The correct orders are exactly as given.
Solution
We note that in a 1‐molal solution, the colligative properties depend on the effective number of solute particles. For each compound the effective van’t Hoff factor (i) is:
- Glucose: i = 1 (non-electrolyte)
- NaCl: i = 2 (100% dissociation into Na⁺ and Cl⁻)
- BaCl₂: i = 3 (100% dissociation into Ba²⁺ and 2 Cl⁻)
- Na₃PO₄: i = 4 (100% dissociation into 3 Na⁺ and PO₄³⁻)
- C₆H₅COOH: i = 0.5 (100% dimer formation so that 1 mole gives 0.5 mole of dimer)
The magnitude of colligative properties (boiling point elevation, freezing point depression, osmotic pressure) is directly proportional to the number of solute particles (moles × i), and the vapour pressure lowering increases with the number of solute particles.
Thus, for a 1‑molal solution:
- Boiling point elevation: Increases with i so the order (lowest BP to highest BP) is
(E) < (A) < (B) < (C) < (D) - Freezing point depression: The depression is greatest for the largest i, so the order (lowest freezing point to highest) is
(D) < (C) < (B) < (A) < (E) - Osmotic pressure: Also directly proportional to i, so the order (greatest to least osmotic pressure) is
(D) > (C) > (B) > (A) > (E) - Vapour pressure: A larger number of solute particles lowers the vapour pressure more, hence (lowest vapour pressure to highest)
(D) < (C) < (B) < (A) < (E)
Thus the correct orders are exactly as given.
