Question
Question: $\xrightarrow{\text{PCl}_5}$X$\xrightarrow[\text{AlCl}_3]{\text{Benzene}}$Y$\xrightarrow{\text{HBr}}...
PCl5XBenzeneAlCl3YHBrZ Major product Z is:
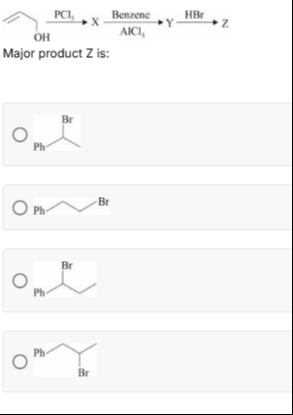
Ph-CH(Br)-CH3
Ph-CH2-CH2-Br
Ph-CH(Br)-CH2-CH3
Ph-CH2-CH(Br)-CH3
Ph-CH(Br)-CH2-CH3
Solution
The reaction sequence can be broken down into three steps:
Step 1: Allyl alcohol PCl5 X
Allyl alcohol is prop-2-en-1-ol, with the structure CH2=CH-CH2-OH.
PCl₅ is a reagent used to convert alcohols into alkyl chlorides. The -OH group is replaced by -Cl.
The reaction proceeds as follows:
CH2=CH-CH2-OH + PCl5 -> CH2=CH-CH2-Cl + POCl3 + HCl
So, product X is allyl chloride (3-chloroprop-1-ene).
Step 2: X BenzeneAlCl3 Y
This is a Friedel-Crafts alkylation reaction. Allyl chloride (CH2=CH-CH2-Cl) reacts with benzene in the presence of AlCl₃ (a Lewis acid catalyst).
First, the alkyl halide reacts with the Lewis acid to form a carbocation:
CH2=CH-CH2-Cl + AlCl3 -> [CH2=CH-CH2]+ [AlCl4]-
The carbocation formed is an allylic carbocation, CH2=CH-CH2+. This carbocation is resonance stabilized:
CH2=CH-CH2+ <-> +CH2-CH=CH2
In Friedel-Crafts alkylation, carbocation rearrangements often occur to form the most stable possible carbocation, and the final product tends to be the most thermodynamically stable one.
When this allylic carbocation attacks benzene, it can potentially form two products:
- Attack at the carbon originally bonded to chlorine (C1):
Ph-CH2-CH=CH2(Allylbenzene) - Attack at the other resonance-stabilized carbon (C3):
Ph-CH=CH-CH3(1-phenylprop-1-ene)
Between allylbenzene and 1-phenylprop-1-ene, the latter is more stable because the double bond is conjugated with the phenyl ring. Furthermore, the Lewis acid (AlCl₃) can catalyze the isomerization of allylbenzene to the more stable 1-phenylprop-1-ene. Therefore, Y is predominantly 1-phenylprop-1-ene.
Structure of Y: Ph-CH=CH-CH3
Step 3: Y HBr Z
This is an electrophilic addition reaction of HBr to the alkene Y (Ph-CH=CH-CH3). This reaction follows Markovnikov's rule, which states that the hydrogen atom of HBr adds to the carbon of the double bond that already has more hydrogen atoms, or, more generally, the addition occurs in such a way as to form the most stable carbocation intermediate.
Let's consider the two possible carbocations formed upon protonation of Ph-CH=CH-CH3:
- If H+ adds to the carbon
CHadjacent to the phenyl group, the carbocation formed isPh-CH2-CH(+)-CH3. This is a secondary carbocation. - If H+ adds to the terminal
CHof the double bond, the carbocation formed isPh-CH(+)-CH2-CH3. This is a secondary benzylic carbocation.
Benzylic carbocations are highly stabilized by resonance with the aromatic ring. Therefore, Ph-CH(+)-CH2-CH3 is the more stable carbocation intermediate.
The bromide ion (Br-) then attacks this carbocation.
So, the major product Z is Ph-CH(Br)-CH2-CH3. This compound is named 1-bromo-1-phenylpropane.
The major product Z is 1-bromo-1-phenylpropane.
