Question
Question: $XO_2^- \rightleftharpoons XO^- \quad E^° = p + q \text{ volts}$ $XO^- \rightleftharpoons \frac{1}{...
XO2−⇌XO−E°=p+q volts
XO−⇌21X2E°=p−2q volts
(Here p, q are positive values)
If XO2−,XO− and X2 are are placed together, then
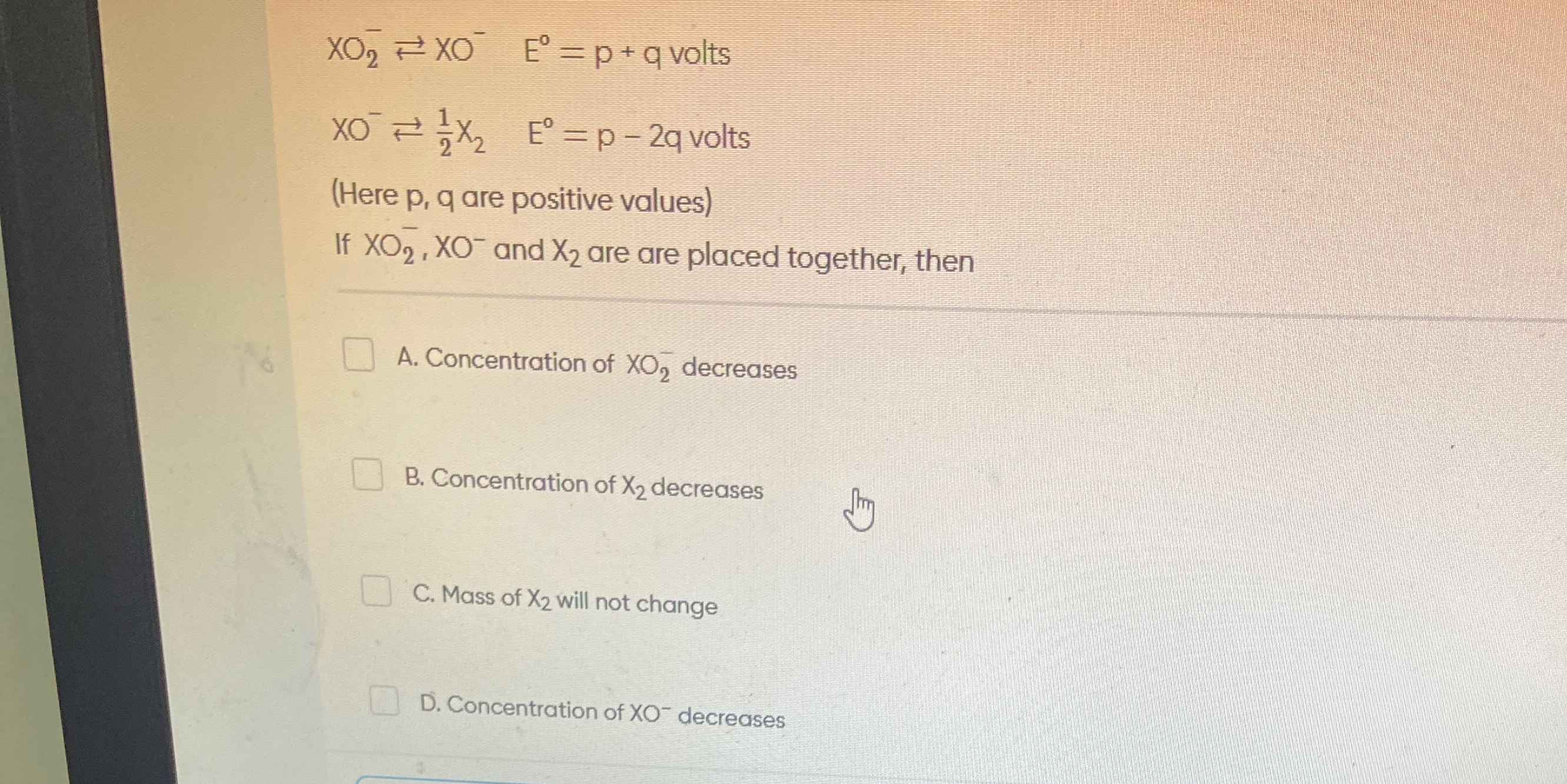
Concentration of XO2− decreases
Concentration of X2 decreases
Mass of X2 will not change
Concentration of XO− decreases
A. Concentration of XO2− decreases
Solution
The given standard electrode potentials are E(XO2−/XO−)=p+q and E(XO−/X2)=p−2q. Since p,q>0, we have p+q>p−2q, meaning E(XO2−/XO−)>E(XO−/X2). This indicates that XO2− is a stronger oxidizing agent than XO− (meaning XO2− readily gets reduced to XO−), and X2 is a stronger reducing agent than XO− (meaning X2 readily gets oxidized to XO−). When XO2−, XO−, and X2 are placed together, the strongest oxidizing agent (XO2−) will react with the strongest reducing agent (X2). The spontaneous reaction will be XO2−+X2→XO− (after balancing). In this reaction, XO2− is a reactant, so its concentration will decrease. X2 is also a reactant, so its concentration will decrease. XO− is a product, so its concentration will increase. Therefore, options A and B are correct. In a single-choice question format, if multiple options are correct, it implies a flaw in the question. However, if a single answer must be chosen, A is a correct statement.
