Question
Question: \(Xe{{F}_{2}}\) is isostructural with: A. \(Te{{F}_{2}}\) B. \(IC{{l}_{2}}\) C. \(SbC{{l}_{2}}...
XeF2 is isostructural with:
A. TeF2
B. ICl2
C. SbCl2
D. BaCl2
Solution
The shape of AB2 molecules with 5 bond pairs and 0 lone pairs is trigonal bipyramidal and with 4 bond pairs and 1 lone pair is seen and with 3 bond pairs and 2 lone pairs is T- shape and with 2 bond pairs and 3 lone pairs is linear. We need to know about VSEPR theory for more details.
Complete step by step answer:
VSEPR theory is defined as the electron pairs surrounding the central atom must be arranged in space as far apart as possible to minimize the electrostatic repulsion experienced between them.
A central atom can be defined as any atom that is bonded to two or more than two other atoms. The first and the most important rule of the VSEPR theory is that the bond angles about a central atom are those that minimize the total repulsion experienced between the Electron pairs in the atom’s valence shell.
Rules:
A lone pair of electrons occupy more space than a bonding pair of electrons because lone pair of electron is under the influence of only one nucleus of the central atom, they are expected to occupy more space with a greater electron density than the bond pair electrons which are under the influence of two nuclei. The decreasing order of repulsion is mentioned below
Lone pair-Lone pair repulsion>Lone pair-Bond pair repulsion>Bond pair- bond pair repulsion
Repulsion forces decrease sharply with increasing interior angle. They are stronger at 90 degree weak at 120 degree and very weak at 180 degree.
Influence of a bonding electron pair decreases with increasing value of electronegativity of an atom forming a molecule.
Multiple bonds behave equivalent to a single electron pair for the purpose of VSEPR bond theory.
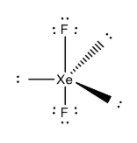
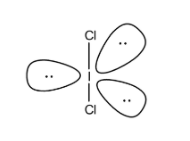
The two electron pair of a double bond occupies more space than one electron pair of a single bond.
The lone pair electrons repels bond pair electrons giving rise to some distortions in the molecular shape.
Now from the above theory and the structure of XeF2, it contains 2 bond pairs and 3 lone pairs as a result from VSEPR theory we can conclude its shape is linear.
Now from the above options ICl2−also contains 2 bond pairs and 3 lone pairs so its shape is also linear. So it is isostructural with XeF2.
Therefore the answer is option B.
Note: Following table shows the relation between BP, LP and shape of a compound.
| Bond pair | Lone pair | Shape |
|---|---|---|
| 5 | 0 | Trigonal bipyramidal |
| 4 | 1 | See saw |
| 3 | 2 | T-shape |
| 2 | 3 | Linear. |
