Question
Question: Xanthoproteic reaction is a reaction of nitric acid with: (a).Vitamins (b).Proteins (c).Enzyme...
Xanthoproteic reaction is a reaction of nitric acid with:
(a).Vitamins
(b).Proteins
(c).Enzymes
(d).Fats
Solution
Focus on the main word given in the question – ‘xanthoproteic’. This reaction is widely used for identification of amino acids.
Complete answer:
The xanthoproteic reaction is a method which is used to detect the presence of protein soluble in a solution, using concentrated nitric acid. It is a qualitative test.
Concentrated nitric acid reacts with proteins to form yellow nitrated products.
Procedure of Xanthoproteic reaction –
add 1 ml of concentrated to 1 ml of test sample
heat the mixture
let the mixture cool
slowly add 40% w/v sodium hydroxide solution until the mixture becomes alkaline and colour change is noted
If the colour of the solution changes from yellow to orange, this indicates the presence of an aromatic amino acid.
Aromatic amino acids, such as Phenylalanine, tyrosine and tryptophan, respond positive to this test. In the presence of concentrated nitric acid, the aromatic phenyl ring is nitrated which gives yellow colored nitro-derivatives. At alkaline pH, the color changes to orange due to the ionization of the phenolic group. The reaction is given below –
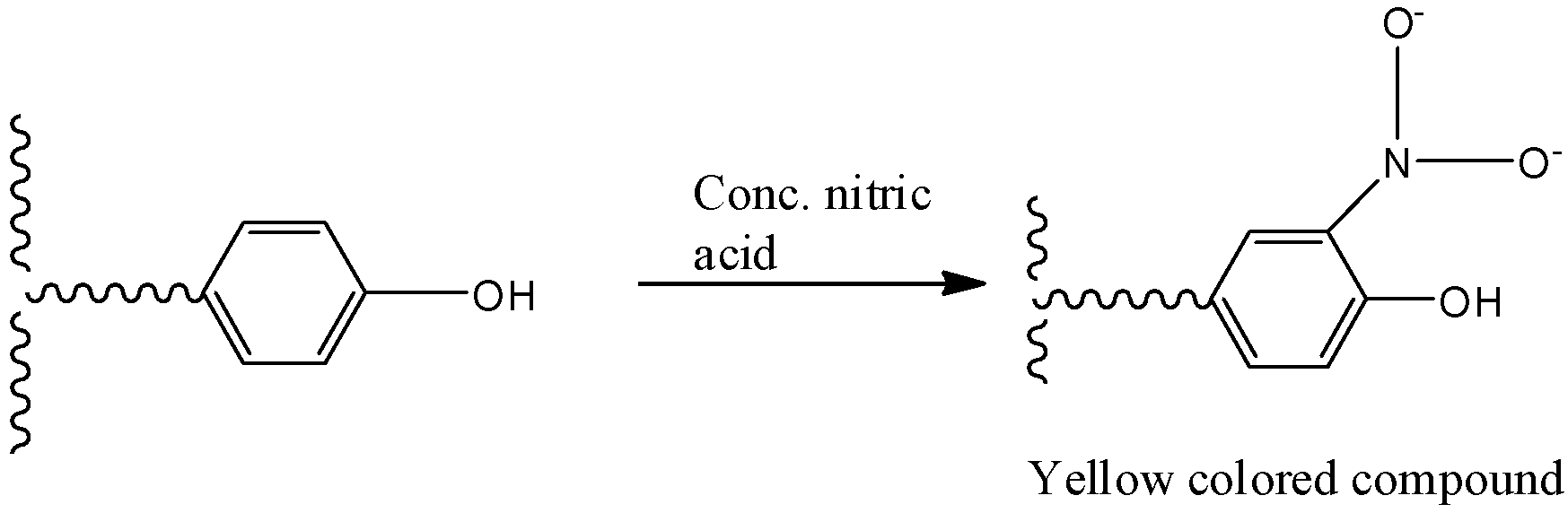
Therefore, the answer is – option (b) – Xanthoproteic reaction is a reaction of nitric acid with proteins.
Note:
Dropping nitric acid on your skin or nails, results in it turning yellow after some time.This indicates the presence of protein on skins and nails. The finger nails show a bright yellow colour, because nails are made up of a protein – keratin. This can be scraped off, but the yellow colour on the skin cannot be scraped off.
