Question
Question: $\newline X^{200} \longrightarrow A^{110} + B^{90}$ If the binding energy per nucleon for X, A and ...
X200⟶A110+B90
If the binding energy per nucleon for X, A and B is 7.4 MeV, 8.2 MeV and 8.2 MeV respectively, what is the energy released?
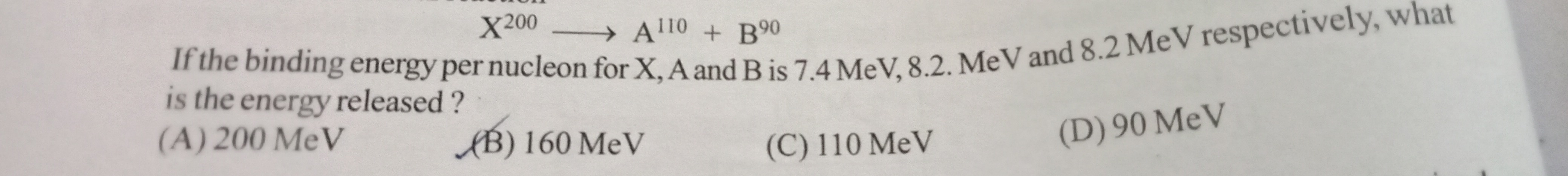
200 MeV
160 MeV
110 MeV
90 MeV
160 MeV
Solution
The energy released in a nuclear reaction is the difference between the total binding energy of the products and the total binding energy of the reactants.
The given nuclear reaction is: X200⟶A110+B90
Where:
- AX=200 (mass number of X)
- AA=110 (mass number of A)
- AB=90 (mass number of B)
The binding energy per nucleon values are:
- BEavg,X=7.4 MeV
- BEavg,A=8.2 MeV
- BEavg,B=8.2 MeV
The total binding energy of a nucleus is calculated as: Total Binding Energy = (Binding Energy per nucleon) × (Mass number)
-
Calculate the total binding energy of the reactant (X): BEX=AX×BEavg,X=200×7.4 MeV=1480 MeV
-
Calculate the total binding energy of the product (A): BEA=AA×BEavg,A=110×8.2 MeV=902 MeV
-
Calculate the total binding energy of the product (B): BEB=AB×BEavg,B=90×8.2 MeV=738 MeV
-
Calculate the energy released (Q): The energy released in a nuclear reaction is given by: Q=(Total Binding Energy of Products)−(Total Binding Energy of Reactants) Q=(BEA+BEB)−BEX Q=(902 MeV+738 MeV)−1480 MeV Q=1640 MeV−1480 MeV Q=160 MeV
Alternatively, we can express the energy released as: Q=(AA×BEavg,A+AB×BEavg,B)−(AX×BEavg,X) Q=(110×8.2 MeV+90×8.2 MeV)−(200×7.4 MeV) Since BEavg,A=BEavg,B=8.2 MeV, we can factor it out: Q=(110+90)×8.2 MeV−200×7.4 MeV Q=200×8.2 MeV−200×7.4 MeV Q=200×(8.2−7.4) MeV Q=200×0.8 MeV Q=160 MeV
The energy released is 160 MeV.
