Question
Question: X-ray diffraction studies show that \(Cu\) crystallises in a face-centred cubic unit cell with an ed...
X-ray diffraction studies show that Cu crystallises in a face-centred cubic unit cell with an edge 3.608×10−8cm. Another experiment shows that Cu is determined to have a density of 8.92gcm−3. Then, what is the atomic mass of Cu?
Solution
The smallest part of a crystal lattice is termed a unit cell. There are three types of cubic unit cell i.e., simple cubic unit, body centred cubic and face centred cubic unit cell. In FCC unit cells, the atoms are present at each corner as well as each face centre of the cube.
Complete answer: A face centred cubic unit cell is represented as follows:
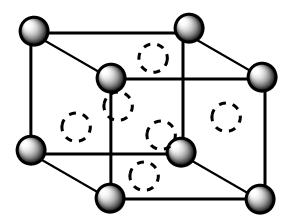
In the structure, the solid spheres denote the atoms present at the corner of the unit cell whereas the dotted sphere denotes the atoms present at the face centre of the unit cell. Total number of atoms in a single FCC unit cell are as follows:
Z=8×81+6×21
⇒Z=4
The density of a unit cell is expressed in terms of edge length, atomic mass, Avogadro’s constant and number of atoms in a unit cell as per following equation:
ρ=NA×a3Z×M−(i)
Where, ρ is the density of unit cell, Z is the total number of atoms in a single unit cell, M is the atomic mass of the element, NAis the Avogadro’s constant and a is the edge length of the unit lattice.
As per question, the given data is as follows:
a=3.608×10−8 cm
ρ=8.92gcm−3
Substituting values in equation (i):
8.92=6.023×1023×(3.608×10−8)34×M
⇒M=42523.35×10−1
⇒M=63.08g
Hence, the atomic mass of copper i.e., Cu is 63.08g.
Note:
It is important to note that each atom present in a unit cell does not completely contribute its each part to a single unit. The corner atoms are shared by eight-unit cells and the atoms present at the face centre are shared by two-unit cells. Hence, in the formula we always use the exact number of atoms that contribute to a cubic unit structure.
