Question
Question: 'X' and 'Y' are two elements which form $X_2Y_3$ and $XY_2$ molecules. If 0.15 mole of $X_2Y_3$ and ...
'X' and 'Y' are two elements which form X2Y3 and XY2 molecules. If 0.15 mole of X2Y3 and XY2 weighs 15.9 gm & 9.3 gm respectively. If oxidation state of 'y' in both compounds is +2 than correct options are:
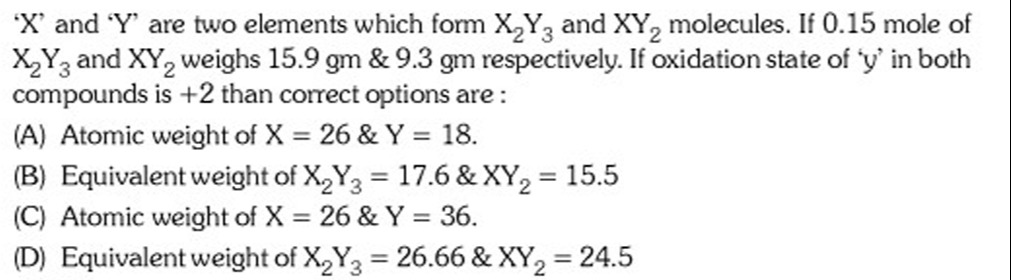
Atomic weight of X = 26 & Y = 18.
Equivalent weight of X2Y3 = 17.6 & XY2 = 15.5
Atomic weight of X = 26 & Y = 36.
Equivalent weight of X2Y3 = 26.66 & XY2 = 24.5
Options (A) and (B) are correct.
Solution
Let the atomic weights of X and Y be A and B respectively.
-
From the compound X2Y3:
- Molecular weight = 2A + 3B
- Given 0.15 mole weighs 15.9 g so: 2A+3B=0.1515.9=106
-
From the compound XY2:
- Molecular weight = A + 2B
- Given 0.15 mole weighs 9.3 g so: A+2B=0.159.3=62
Solving the equations:
Subtracting 2 times the second equation from the first equation: (2A+3B)−2(A+2B)=106−2(62) 2A+3B−2A−4B=106−124 −B=−18 B=18
Plugging B = 18 into the second equation: A+2(18)=62 A+36=62 A=26
Therefore, the atomic weight of X is 26 and the atomic weight of Y is 18.
Equivalent Weight Calculation:
Given that the oxidation state of Y is +2:
-
For X2Y3:
- Total oxidation by Y = 3 * (+2) = +6
- So X must be -3 each (since 2*(-3) + 6 = 0)
- Total electrons involved per molecule = 2 * 3 = 6
- Equivalent weight = 6Molecular weight=6106≈17.67≈17.6 g/equivalent
-
For XY2:
- Oxidation: Let X be x; then x + 2*(+2) = 0 gives x = -4
- Total electrons involved per molecule = 4
- Equivalent weight = 4Molecular weight=462=15.5 g/equivalent
Thus, the atomic weights are X = 26 and Y = 18, and the equivalent weights are as calculated.
