Question
Question: Write the structures of the main products of the following reactions. 
Solution
Primary, secondary, and tertiary amines are traced and discerned using Hinsberg test. The given reactant is benzene sulfonyl chloride which is known as Hinsberg reagent. Along with the Hinsberg reagent, aqueous KOH is also added. So based on the solubility of the product formed, three of them can be differentiated.
Complete step by step solution:
Amines are organic compounds having one or more nitrogen groups. Amines are divided into primary, secondary, tertiary, and aromatic amines. Primary, secondary, tertiary amines are detected and distinguished by the Hinsberg test. This was introduced by Oscar Heinrich Daniel Hinsberg.
Hinsberg reagent is the benzene sulfonyl chloride, PhSO2Cl. Benzene sulfonic acid or its salt is chlorinated with phosphorus oxychloride which produces benzene sulfonyl chloride.
PhSO3−Na+PCl5POCl3PhSO2Cl
When primary amine is reacted with Hinsberg reagent, it forms a sulfonamide which on reaction with base gives sulfonamide salt. It is water-soluble.
PhSO2N(H)R+NaOH→Na+[PhSO2NR−]+H2O
Tertiary amines can react with Hinsberg reagent under certain conditions only. Reaction speed, concentration, solubility and temperature have to be considered.
PhSO2Cl+R3N+H2O→R3NH+[PhSO3−]+HCl
This also gives water-soluble sulfonate salts.
This benzene sulfonyl chloride is reacted with the amine C3H9Nin the presence of aqueous KOH. It should be shaken well.
There is a direct formation of an alkali insoluble sulfonamide when secondary amine is treated with Hinsberg reagent. The reaction of benzene sulfonyl chloride is reacted with secondary amine, (CH3)2NH to give the following products:
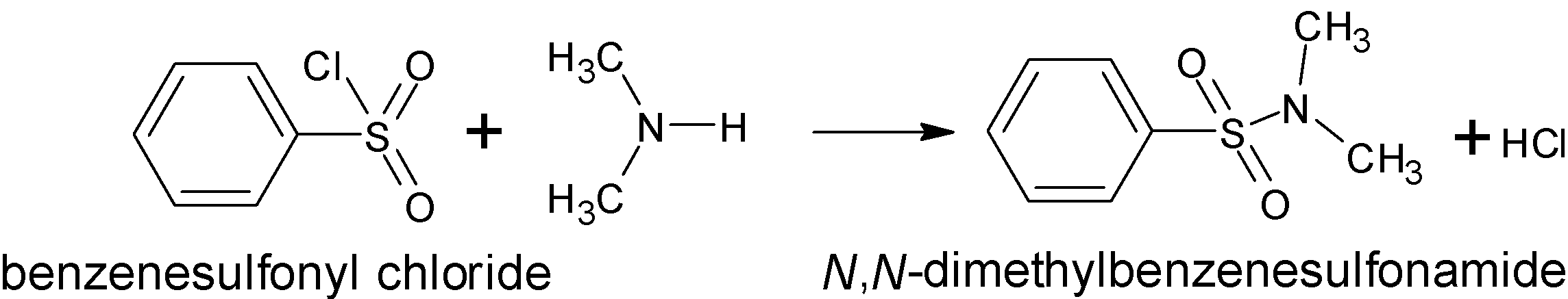
Thus the product formed is N,N-dimethyl benzene sulfonamide.
Note:
Sulfonyl chloride undergoes hydrolysis when reacted with tertiary amines which produces water soluble sulfonate salts. Differentiation of primary, secondary, tertiary amines are based on solubility of the product in alkali.
