Question
Question: write the structures of the following compounds: (a) Prop-1-ene (b) 2, 3-dimethylbutane (c) 2-me...
write the structures of the following compounds:
(a) Prop-1-ene (b) 2, 3-dimethylbutane
(c) 2-methyl propane (d) 3-hexene
(e) prop-1-yne (f) 2-methyl prop-1-ene
(g) Alcohol with molecular formula C4H10O
Solution
Structure of any compound is the depiction of its IUPAC name. The IUPAC has set certain norms for the naming of organic compounds, these norms also affect the structures of organic compounds. Here are various structures of organic compounds.
Complete answer:
(a)Prop-1-ene: this compound has the molecular formula C3H6, as it is an alkene, therefore a double bond is present in propene. The structure is,
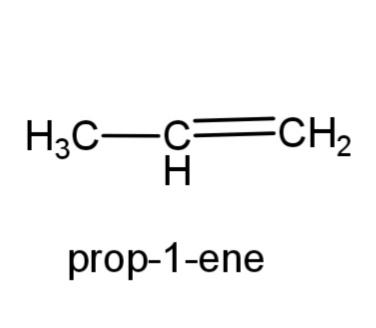
(b) 2, 3-dimethylbutane- this compound consists of a butane carbon chain with methyl group at 2 and 3 carbons. The structure is,
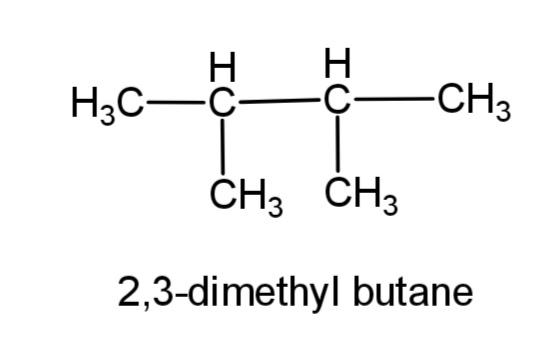
(c) 2-methyl propane – this compound consists of a 3 carbon propane chain with methyl group at carbon-2. The structure is,
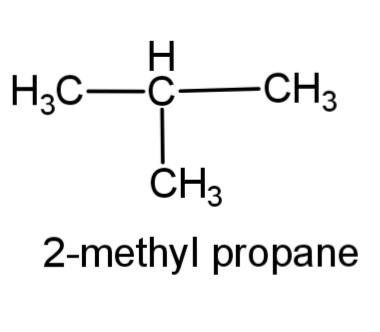
(d) 3-hexene – this compound consist of a 6 carbon chain, with a double bond at carbon 3, the structure is,
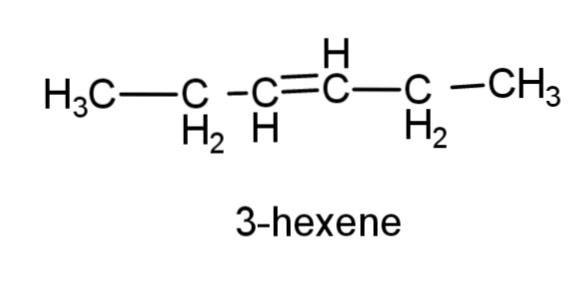
(e) prop-1-yne- this compound is an alkyne with triple bond at carbon 1, the structure is,
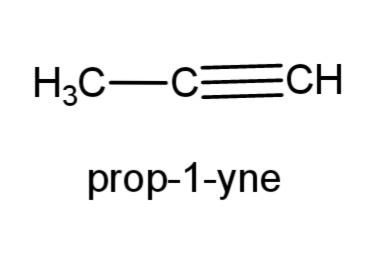
(f) 2-methyl prop-1-ene- this compound consist of a propene, with a methyl group at carbon-2, the structure is,
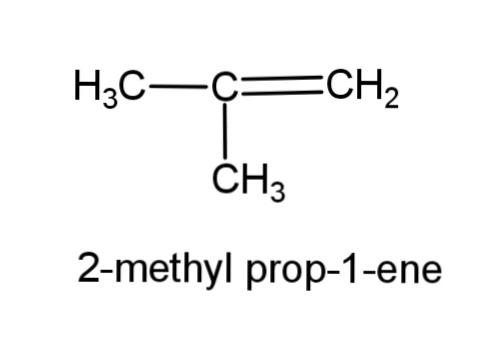
(g) Alcohol with molecular formula C4H10O - there can be 3 different structures of alcohol with this molecular formula, the structures are,
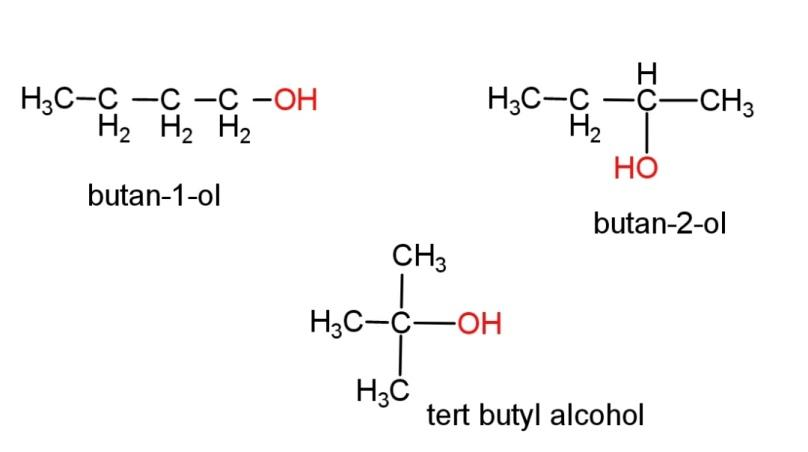
Hence, all the structures have been shown above.
Note:
butan-2-ol consist of a chiral center, where all the 4 attached groups on the carbon atom are different. The chiral carbon is the second carbon. Chirality in a molecule results in optical rotation of the molecule with plane polarized light (PPL).
