Question
Question: Write the structures of the following compounds: A.2-chloro, 3-methyl pentane B.p-bromo chloro b...
Write the structures of the following compounds:
A.2-chloro, 3-methyl pentane
B.p-bromo chloro benzene
Solution
One can solve this question by keeping in mind the IUPAC nomenclature rules. Parent carbon chain can be determined by calculating the number of carbons in the longest carbon chain. One or more functional groups attached to it can be named as per priority and alphabetic order.
Complete step by step answer:
-If organic compounds contain one principal functional group then the longest continuous chain of carbon atoms having that functional group is selected. Functional groups are specific substituents in the molecules which are responsible for the characteristic chemical reactions of those molecules.
-The IUPAC nomenclature of organic chemistry is a system of organic chemical compounds in nomenclature of chemicals as stated by the International Union of Pure and Applied Chemistry. Informally we call it the Blue Book. The book mentions certain rules for naming of compounds having functional groups.
-As per IUPAC rules, the first thing is to select the longest continuous chain. Then this chain is numbered and the numbering should begin from the end which is close to the highest priority group called as principal functional group. Each group has a certain assigned name whose numbering depends on the position of the carbon it has been attached. These groups other than the principal functional group are generally arranged alphabetically.
-In (i), 2-chloro, 3-methyl pentane, it is an aliphatic compound with root word pentane i.e. 5 carbon chains. Its second position is occupied by the chloro group and third position by methyl group. The structure thus formed is
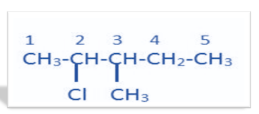
In (ii) p-bromo chloro benzene, it is an aromatic compound which has a benzene ring. The first position on benzene is given to the chloro group and to its para position i.e. fourth position, bromo group is attached. The structure thus formed is
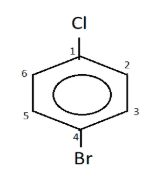
Note:
One must remember that every substituent should have a number and in case of aromatic compounds, the positions with respect to the first one are known as ortho (1,2 ; 1,6), meta(1,3 ; 1,5) and para(1,4). Use of commas to separate numbers and hyphens to separate numbers and substituents is done while writing the formula.
