Question
Question: Write the structures of following alkanes: (A) \[5-\left( 2-Ethylbutyl \right)-3,\text{ }3-dimethy...
Write the structures of following alkanes:
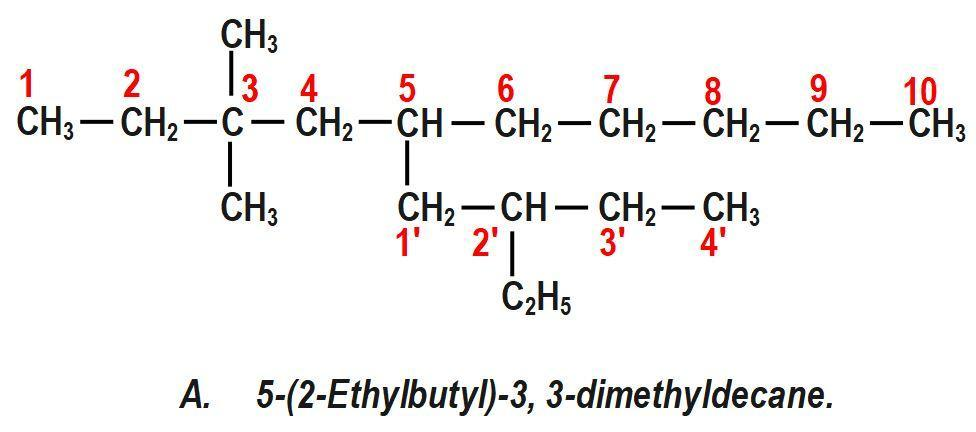
(A) 5−(2−Ethylbutyl)−3, 3−dimethyldecane.
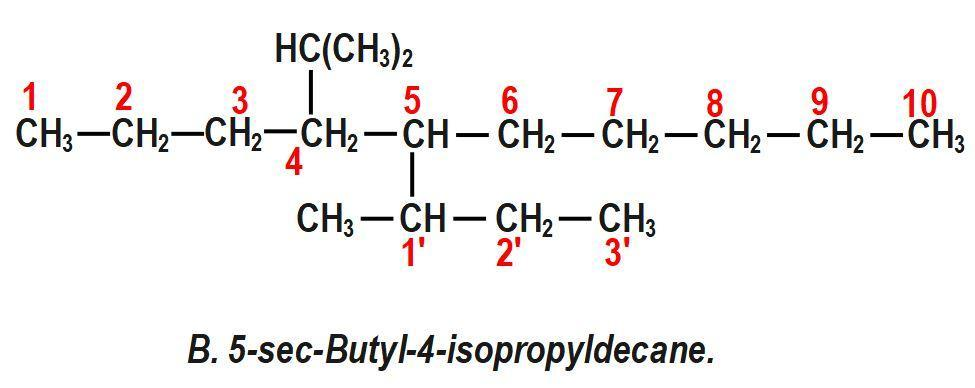
(B) 5−sec−Butyl−4−isopropyldecane.
(C) 2, 3−Dimethyl−6−(2−methylpropyl) decane.
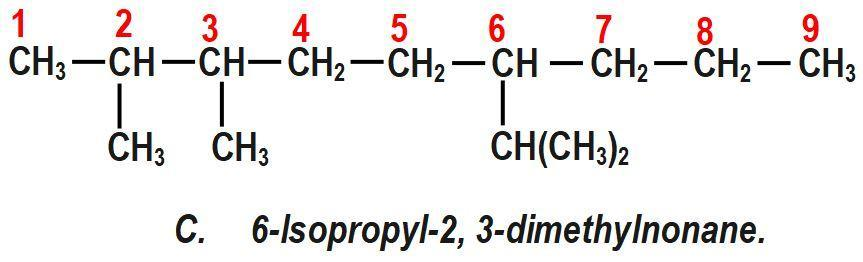
(D) 6−Isopropyl−2, 3−dimethylnonane.
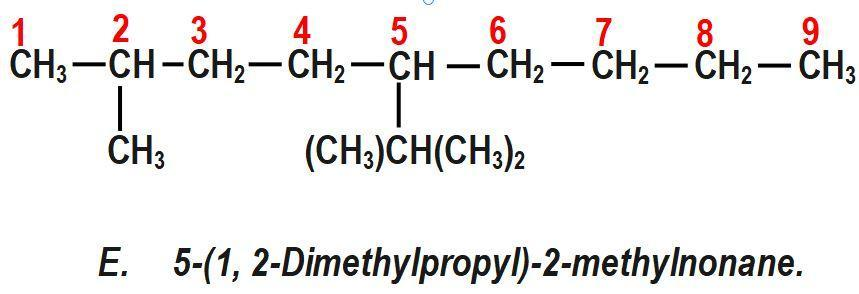
(E) 5−(1, 2−Dimethylpropyl)−2−methylnonane.
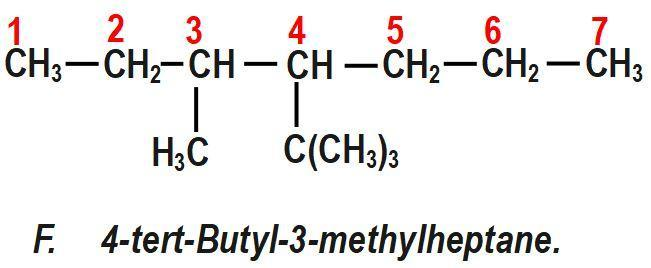
(F) 4−tert−Butyl−3−methylheptane.
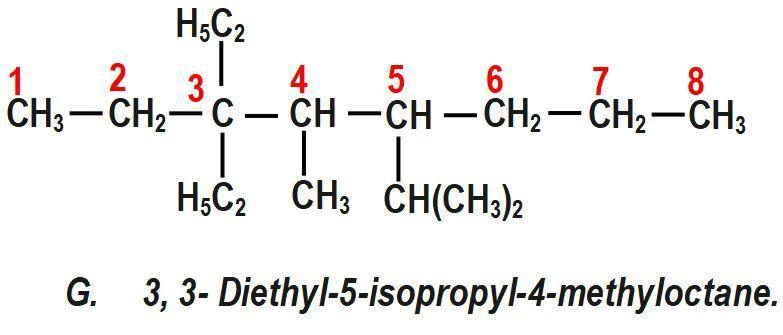
(G) 3, 3− Diethyl−5−isopropyl−4−methyloctane.
Solution
Hint : We know that the alkanes are organic compounds composed of single-bonded carbon and hydrogen atoms; the most basic family of compounds has been called alkanes. They comprise only hydrogen and carbon. Each carbon atom creates four bonds, and each hydrogen atom generates a solid bond.
Complete Step By Step Answer:
If organic compounds contain one principal functional group then the longest continuous chain of carbon atoms having that functional group is selected. Functional groups are specific substituents in the molecules which are responsible for the characteristic chemical reactions of those molecules. As per IUPAC rules, the first thing is to select the longest continuous chain. Then this chain is numbered and the numbering should begin from the end which is close to the substituent groups. Each substituent group has a certain assigned name whose numbering depends on the position of the carbon it has been attached to. These groups are generally arranged alphabetically.
The IUPAC nomenclature of organic chemistry is a system of organic chemical compounds in nomenclature of chemicals as stated by the International Union of Pure and Applied Chemistry. Informally we call it the Blue Book. The book mentions certain rules for naming compounds having functional groups. Chemists have been using the line-angle formulas since it is faster and easier to draw, unlike condensed structural formulas.
Note :
Remember that while drawing the structure of any compound, see that the valency of each element is satisfied. The valency of elements which are not attached to any molecule is satisfied by attaching hydrogen atoms to them. In case of alkenes and alkynes double and triple bonds respectively are used.
