Question
Question: Write the structure of the major organic product isolated from the reaction of 3-hexyne with chlorin...
Write the structure of the major organic product isolated from the reaction of 3-hexyne with chlorine (1 mol) :
A. trans-3,4-dichloro-3-hexene
B. cis-3,4-dichloro-3-hexene
C. trans-2,4-dichloro-3-hexene
D. cis-2,4-dichloro-3-hexene
Solution
The addition of chlorine atom to hydrocarbons is called chlorination. In the chemical reaction of chlorine always the addition of halogen to the triple bonds will be in trans direction due to an electron rich environment.
Complete answer:
- In the question it is asked to draw the structure of a major organic product that will be going to form when I mole of chlorine is going to react with 3-hexyne.
- First we know the structure of the organic compound 3-hexyne.
- The structure of 3-hexyne is as follows.
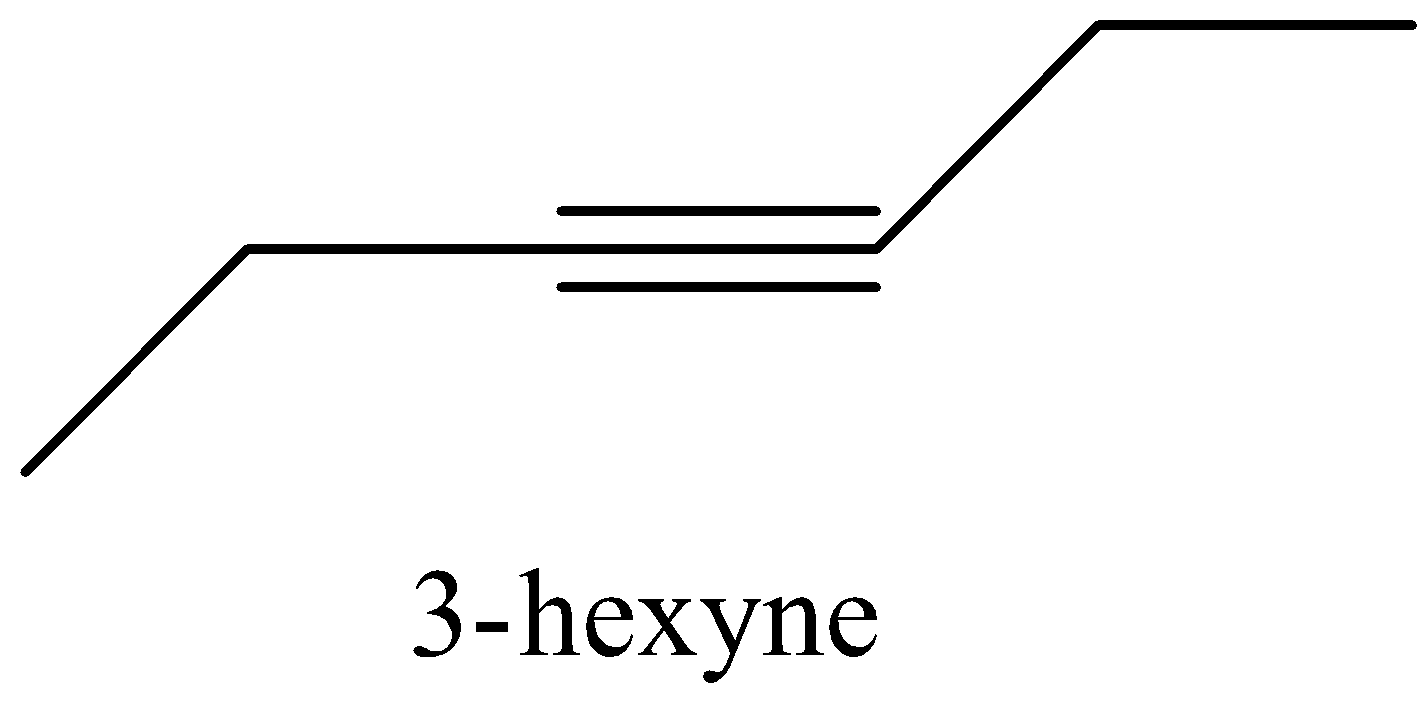
- There are six carbon atoms present in the structure of 3-hexyne and there is a triple bond at the middle of the organic compound 3-hexyne.
- The chemical reaction of 3-hexyne with one mole of chlorine is as follows.
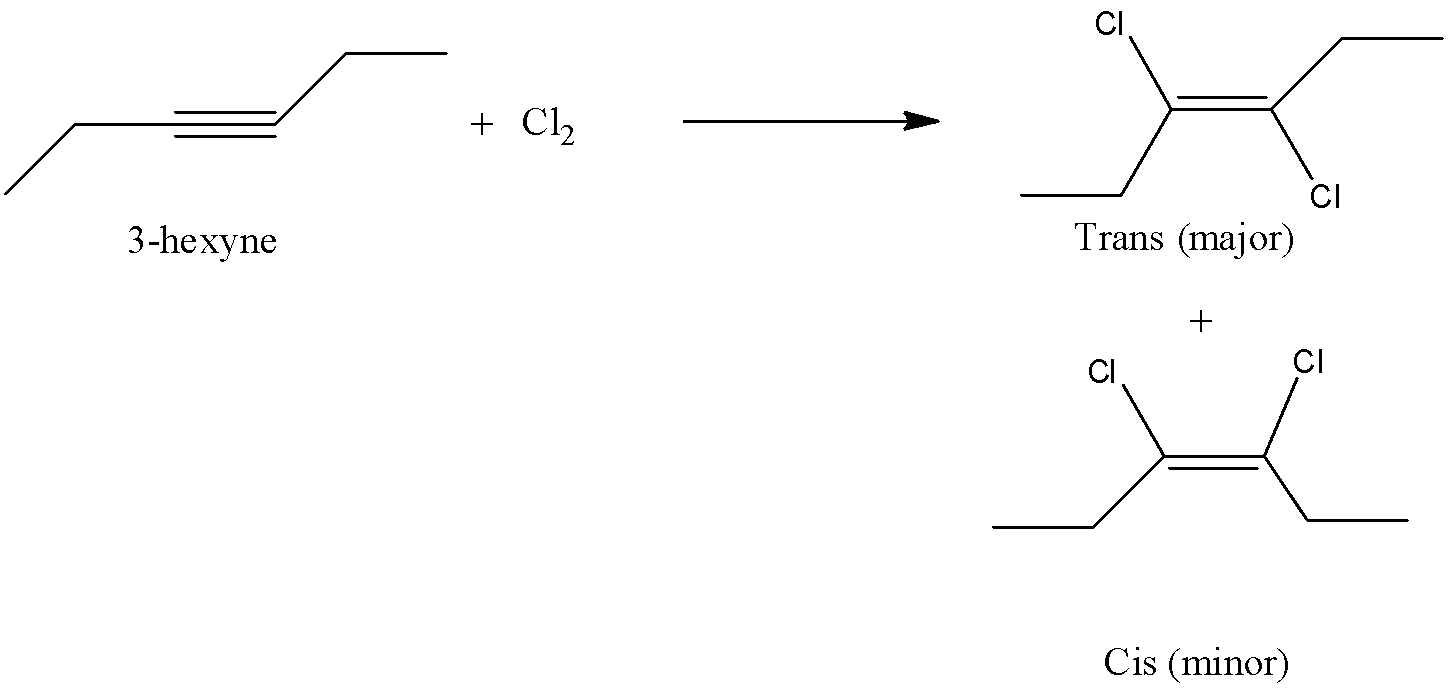
- In the above chemical reaction 3-hexyne is going to react with chlorine and forms two products in different ratios.
- The two products are trans-3,4-dichloro-3-hexene and cis-3,4-dichloro-3-hexene in different ratios.
- The major product formed by the reaction of 3-hexyne with one mole of chlorine is trans-3,4-dichloro-3-hexene.
Therefore the correct option is A.
Note:
Addition reaction of chlorine on 3-hexyne is an example of electrophilic addition chemical reaction. Always high stable and less energy containing molecules are going to form in any organic chemical reaction.
