Question
Question: Write the structure of the following IUPAC name 2-methoxybutan-1-ol....
Write the structure of the following IUPAC name 2-methoxybutan-1-ol.
Solution
The root word gives the longest carbon chain and then attach the substituents as per the positions given in the IUPAC name.
Complete step by step answer:
-According to IUPAC nomenclature, if organic compounds contain one principal functional group then the longest continuous chain of carbon atoms having the principal functional group is selected. In organic chemistry, functional groups are specific substituents in the molecules which are responsible for the characteristic chemical reactions of those molecules.
-The word root for the given compound is butane. A hydroxyl group is attached at the first position of butane and one methoxy group on the second position of butane. So, the prefix will be ‘2-Methoxy’. As there are two functional groups present- ether and alcohol, alcohol has higher priority as per IUPAC rules. Therefore, the numbering will start from the carbon attached to the hydroxyl group.
-Also, the name of the compound is always written with the substituents in the alphabetical order followed by the base name which is derived from the number of carbons in the parent chain. The suffix of the name shows the type of functional group present on parent chain with higher priority.
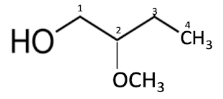
-Hence, the structure of the following IUPAC name 2-methoxybutan-1-ol can be:
Note:
Functional groups should get the least number while numbering the longest carbon chain. If there are more than one functional group, the one with higher priority should get the least number. For example, carboxylic acid has higher priority than hydroxyl groups.
