Question
Question: Write the structure of possible major monosubstituted products formed when \(B{{r}^{+}}\) attacks th...
Write the structure of possible major monosubstituted products formed when Br+ attacks the following molecules. Justify your answer.
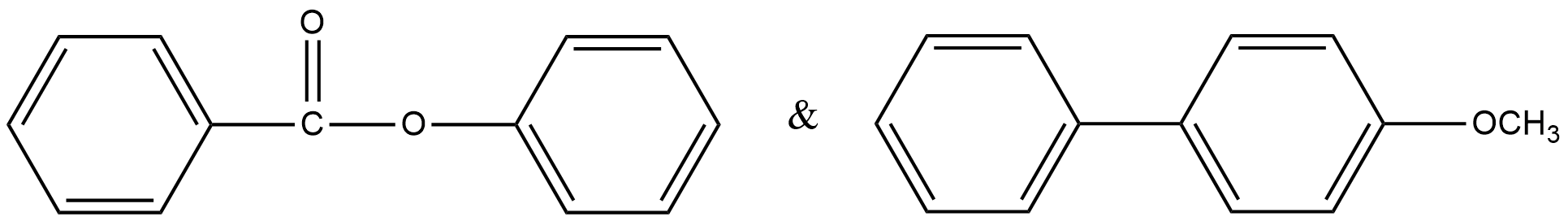
Solution
Br+ is an electrophile, which means going to attach on the carbon where electron density is very high. Means Br+ is going to participate in electrophilic substitution chemical reaction and gives bromo derivative as the product.
Complete answer:
- In the question it is asked to write the of possible major monosubstituted products formed when Br+ attacks the given molecules.
- First, we have to find the electron rich centers in the given molecules and they are as follows.
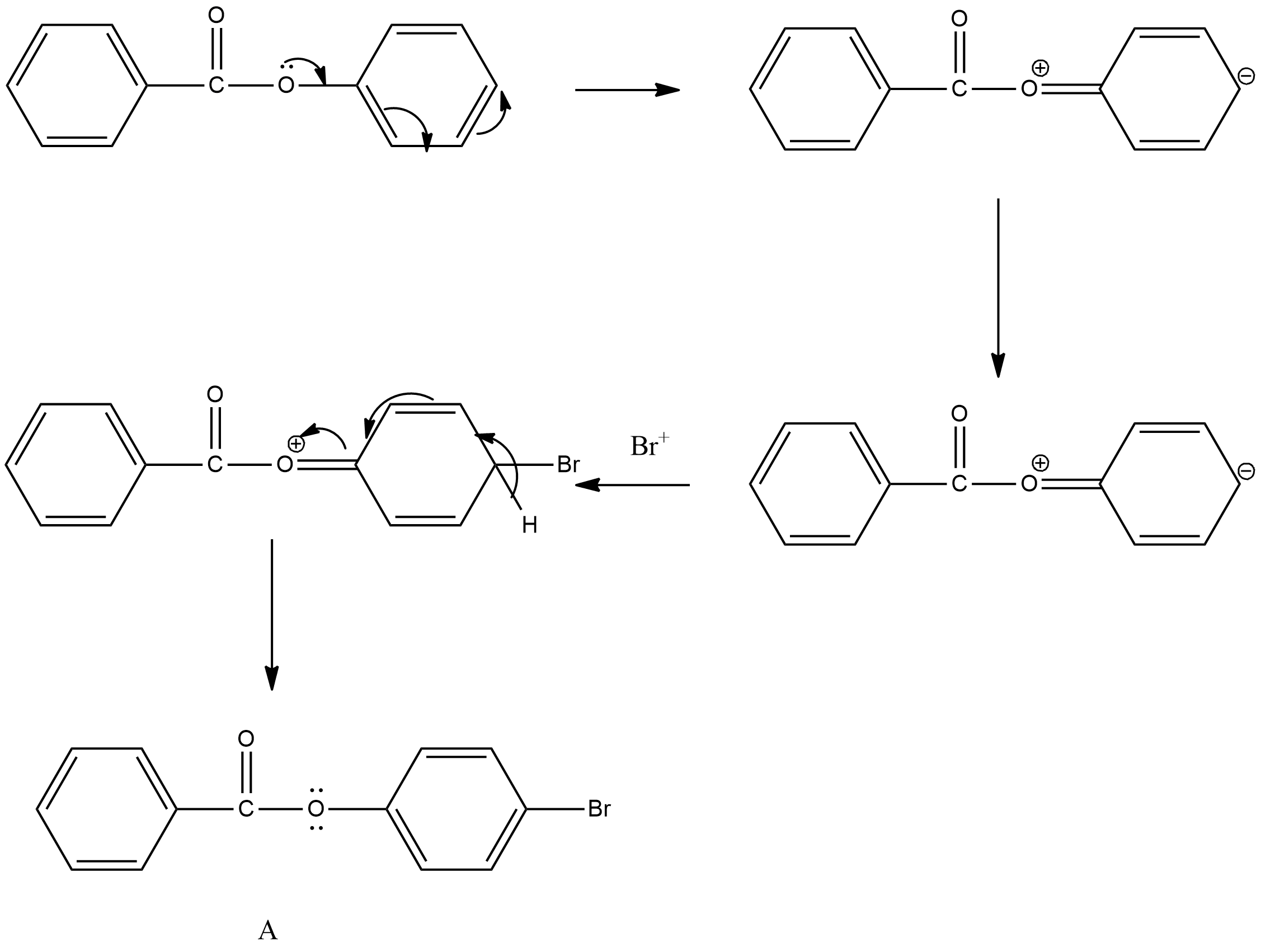
- In the above chemical reaction we can see that the electrons which are present on the oxygen atom of the ester functional group are going to participate in resonance with the benzene ring and generate a negative charge on the benzene ring.
- The electrophilic bromine cation is going to attack on the electron rich carbon of the chemical and we can see the proper mechanism of attack of the bromine cation in the above chemical reactions.
- Coming to the attack of the bromine cation on the second molecule and it is as follows.
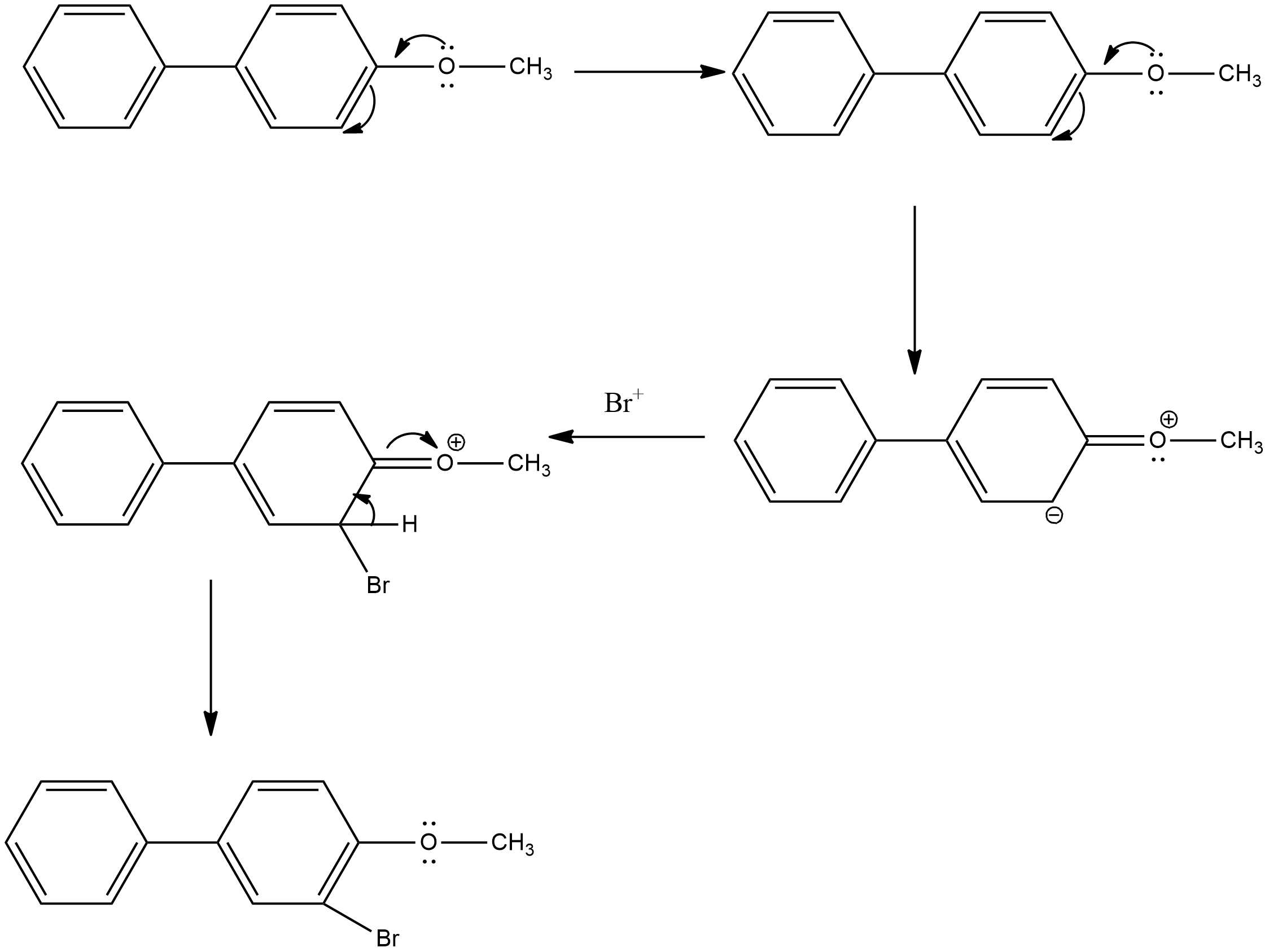
- In the above chemical reaction we can see that the bromine cation is going to react with electrophilic carbon which is generated due to the presence of the methoxy group on one of the benzene rings.
- Therefore, the formed monosubstituted products of the given chemicals are as follows.
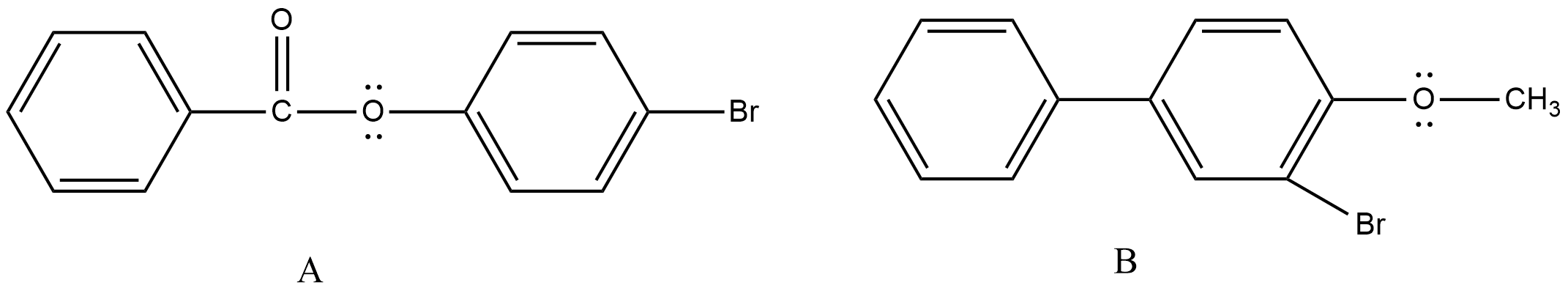
Note:
The electron donating group directs the electrophilic bromine cation to the carbon which is going to have an electron rich environment. If electron withdrawing groups are present in the molecules, then the bromine cation is not going to participate in the chemical reaction.
