Question
Question: Write the structure of oleum (\({H_2}{S_2}{O_7}\))...
Write the structure of oleum (H2S2O7)
Solution
Oleum is also known as pyrosulfuric acid and disulfuric acid. The IUPAC name of oleum is sulfo hydrogen sulphate.
Complete step by step answer:
Oleum is the main constituent of fuming sulfuric acid. Sulfuric acid is a stronger acid than pyrosulfuric acid. Salts such as sodium and potassium pyrosulfate are obtained by reacting them with the bases.
This acid can also be synthesised by reacting excess SO3 with sulfuric acid.
You can see the structure of oleum in the below diagram:
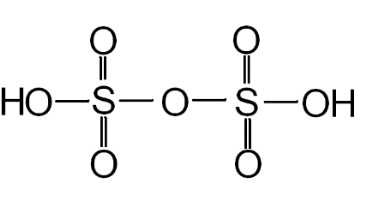
The number of S-S bonds present in pyrosulphuric acid is zero. There are a total of 4 double bonds in the structure. We can see that four oxygen atoms are linked to S by a double bond and the rest by a single bond.
Properties of oleum:
- The property value of hydrogen bond donor is 2 and that of the hydrogen bond acceptor is 7. The rotatable bond count is 7.
- The molecular weight of oleum is 178.129g/mol.
- It has a melting point of 36∘C.
- Disulfate is the conjugate base of oleum.
- The chemical formula of oleum is H2S2O7
We have seen the structure and properties of oleum; now let us see where it is used.
Applications of Oleum
- Oleum is used in the manufacture of explosives and dyes.
- It is also used as a sulfating agent.
- It is used in petroleum refining applications.
It can cause severe eyes irritation when exposed to it and permanent tissue damage when swallowed.
Note: Do not confuse pyrosulfuric acid (H2S2O7) with peroxymonosulfuric acid. The chemical formula for peroxymonosulfuric acid is H2S2O5.
