Question
Question: Write the structure of nitrolic acid....
Write the structure of nitrolic acid.
Solution
Hint: Nitrolic acid is a weak acid, which is prepared by the action of nitrous acid on any nitrile source.
Complete step by step answer:
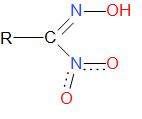
Nitrolic acid is a compound with a general formula RC(NO2)=NOH, where R can be any group. It is a weak acid and has a strong conjugate base. It is not a very popular compound and has been formed in laboratories out of curiosity.
Nitrolic acid is prepared by the action of nitrous acid - HNO2 on primary nitroparaffins - RCH2NO2(a nitrile source) and that react with an alkali to give intensely red-colored solutions of their salts. The sample reaction is as follows –
RCH2NO2+HNO2→RC(NO2)=NOH+H2O
This reaction was first demonstrated by Victor Meyer using a compound named - nitroethane. This reaction proceeds via attack on the nitronate anion (nitronate anion is formed as an intermediate in this reaction).
The structure of nitrolic acid is as given below –
Additional Information:
Nitrous acid - HNO2 is a monobasic weak acid. Nitrous acid is also used to distinguish primary, secondary and tertiary amines.
Note: Pseudo Nitrile is a class of compounds with the general formula RR′C(NO)NO2. It is formed by the action of nitrous acid on a disubstituted nitromethane RR′CHNO2 . It occurs as a pungent, colorless, solid dimers which when fused or dissolved, it depolymerizes into the monomers of intense and characteristic blue color. The reaction for preparation of Pseudo Nitrile is the same as that of Nitrolic acid –
RR′CHNO2+HNO2→RR′C(NO)NO2=NOH+H2O
