Question
Question: Write the structure of \[{{H}_{2}}S{{O}_{4}}\]....
Write the structure of H2SO4.
Solution
Hint: We should know that H2SO4 is a mineral acid composed of the elements sulphur, oxygen and hydrogen. It is a colourless, odourless, and viscous liquid that is soluble in water and we use it synthesized in reactions that are highly exothermic. It is known by the name of sulphuric acid.
Step by step answer:
We know that H2SO4 is sulphuric acid.
Sulphur is the centre atom, which is surrounded by O atoms.
Now, we have H atoms and we will place them in the structure.
We know how to find valence electrons for any element. So, we will now find valence electrons for hydrogen, sulphur and oxygen.
Valence electron for H: Hydrogen has atomic number one. So, it will have one electron in one and only orbit. It needs one electron to fill its shell so its valency is one.
Valence electron for O: Oxygen has atomic number eight. It has 6 electrons in the outer shell. It needs two more electrons to fill its shell so its valency is 2 and the number of valence electrons is six.
Valence electron for S: Sulphur has atomic number 16. It also has 6electrons in the outer shell. So, it has six valence electrons.
Now, we will indicate the number of electrons. In the first step we should add up the valence electrons in each element. We know that H has 1, S has 6, and O also has 6. Therefore,(2×valence for H) + ( valence for S) + (4×valence for O),
= 2(1) + (6) + 4(6) = 32 valence electrons
Divide this number by 2, we get 16 electron pairs, which are either shared by bonded elements or are lone pairs in the Lewis Structure
Now, sulphur will form a double bond with oxygen.
The structure should look like this. The lines are electron pairs, and a pair of dots is also electron pairs. If you count the number of electrons pairs, you should see 16 of them. Hydrogen can have a maximum of 2 electrons, while the other elements can have a max of 8.
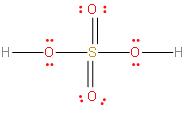
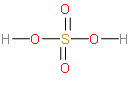
The H2SO4 structure will look this.
Note: We should note that sulphuric acid is one of the most important industrial chemicals. Sulphuric acid used by us is in the production of fertilizers. Other chemicals produced like hydrochloric acid, nitric acid, synthetic detergents, dyes and pigments, explosives, and drugs are possible because of sulphuric acid. And one important use is that it is used in petroleum refining.
