Question
Question: Write the structure of following alkane. 6-Isopropyl -2,3-dimethylnonane....
Write the structure of following alkane.
6-Isopropyl -2,3-dimethylnonane.
Solution
Alkanes are saturated hydrocarbons with general formula CnH2n+2. Where, C is carbon, H is hydrogen and n is the number of carbon atoms in the compound.
Complete step by step answer:
We will solve this question step by step.
(1) In the first step identify the parent alkane. Here parent alkane is nonane which has 9 Carbon atoms in a straight longest chain. So we will first draw 9 carbon atoms in a straight chain.
C - C - C - C - C - C - C - C - C
(2) Now will start numbering the C-atoms either from left or right. Here we start numbering from left side
123456789
C−C−C−C−C−C−C−C−C
(3) Now put substituents at the respective position. Here substituents are 2 methyl groups and one isopropyl group. Methyl group, CH3 is present at 2 and 3 position and isopropyl group, CH(CH3)2 present at 6 position.
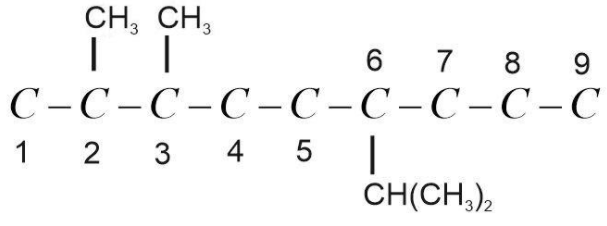
Now balance the remaining valency of C-atoms by attaching H-atoms with it. Valency of C-atom is 4 and the valency of H-atom is 1. It should be satisfied while drawing the structure.
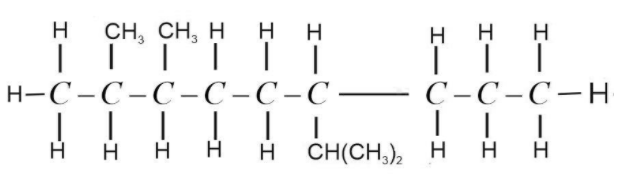
This is the complete structural formula of 6-Isopropyl -2,3-dimethylnonane. It is not always necessary to show the H-atom. We can assume the presence of H-atom and write normal structure formula as,
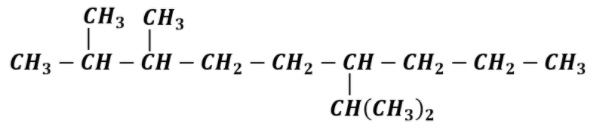
This is the required structure 6-Isopropyl -2,3-dimethylnonane.
Note: While drawing the structure of any compound, see that the valency of each element is satisfied. The valency of elements which are not attached to any molecule is satisfied by attaching H-atoms to them. In case of alkenes and alkynes double and triple bonds respectively are used.
