Question
Question: Write the structure of diborane and explain the nature of bonding in it....
Write the structure of diborane and explain the nature of bonding in it.
Solution
The chemical compound diborane(6), also known as diborane, has the formula B2H6 and is made up of boron and hydrogen. It's a colourless, pyrophoric gas with an odiferous sweet odour. Boron hydride, diboron hexahydride, and bromoethane are all synonyms. Diborane is a common boron compound with a wide range of uses. Its electronic structure has gotten a lot of interest. It has a number of derivatives that are valuable reagents.
Complete answer:
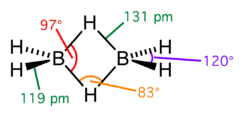
The structure of the Diborane molecule is made up of four hydrogen atoms and two boron atoms that are aligned in the same plane as seen in the diagram. The boron atom has four hybrid orbitals and is sp3 hybridised. Three of the four hybrid orbitals have one electron apiece, while the fourth is an empty orbital. As a result, diborane is an electron-poor component. The connection between the boron atoms and the bridging hydrogen atoms, on the other hand, is not the same as in hydrocarbon molecules. After connecting to the terminal hydrogen atoms with two electrons, each boron has one valence electron left for further bonding.
Each of the bridging hydrogen atoms contributes one electron. As a result, four electrons hold the B2H2 ring together, which is an example of 3 centre 2 centre electric bonding. A 'banana bond' is a term used to describe this sort of relationship.
The lengths of the BH bridge and BH terminal bonds are 1.31 and 1.19 A, respectively, and this disparity in lengths reflects the strength differences between these bonds, with the BH bridge bonds being considerably weaker.
Hence option A is correct.
Note:
A bent bond, sometimes known as a banana bond, is a kind of covalent chemical connection with a geometry that resembles that of a banana in organic chemistry. The phrase is a generic description of electron density or configuration that resembles a similar "bent" structure inside tiny ring molecules like cyclopropane, or as a representation of double or triple bonds within a compound that is an alternative to the sigma and pi bond model.
