Question
Question: Write the structure of chlorous acid \(\left[ {HOClO} \right]\) ....
Write the structure of chlorous acid [HOClO] .
Solution
Chlorous acid is an inorganic compound with formula HClO2 . It is a weak acid. Chlorine has oxidation state +3 in this acid. It consists of one chlorine atom, one hydrogen atom, and two oxygen atoms.
Complete step by step answer:
The structure of chlorous acid is as follows:
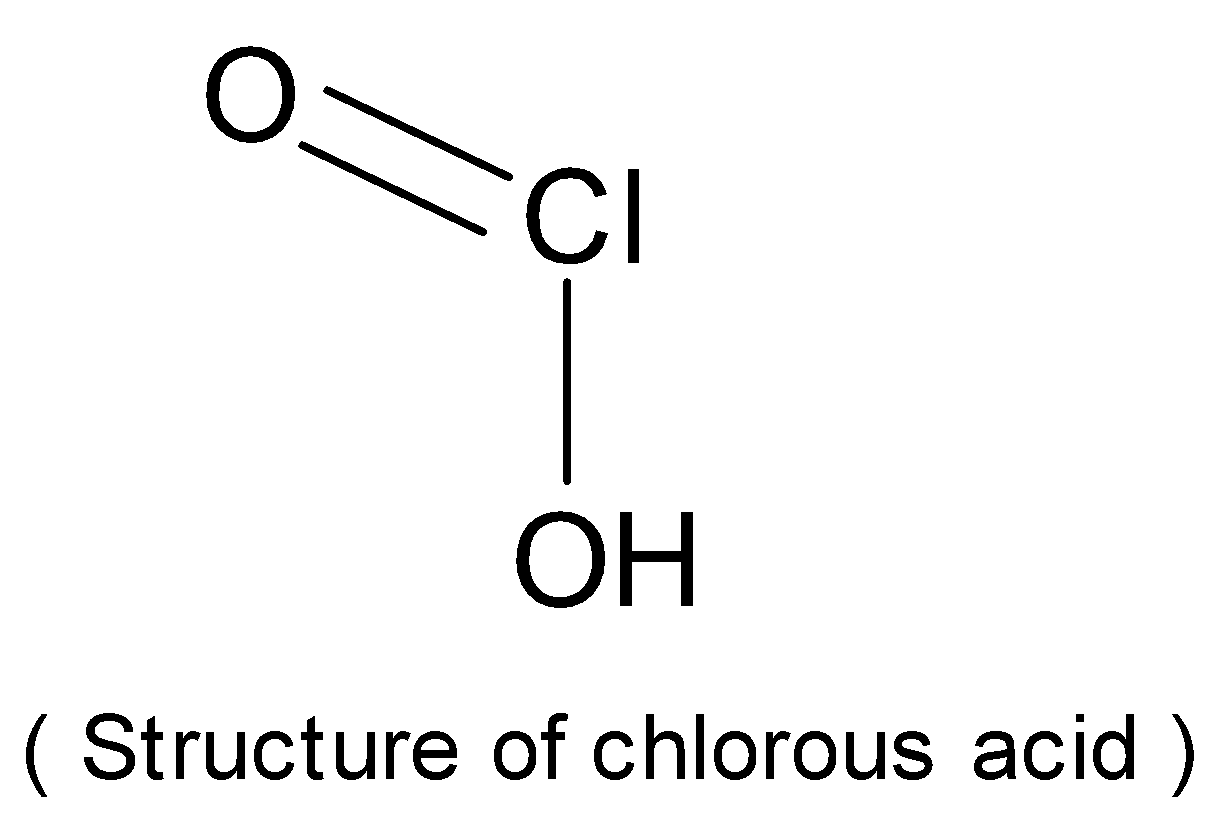
Chlorous acid is a chlorine oxoacid. It is a conjugate acid of chlorite.
The pure chlorous acid is unstable, disprotionating to hypochlorous acid ( Cl oxidation state of +1 ) and chloric acid ( Cl oxidation state of +5 ).
2HClO2→HClO+HClO3
The acid is difficult to obtain in pure substances; the conjugate base, chlorite, derived from this acid is stable.
One example of a salt of this anion is well known sodium chloride.
This and salts related to this are sometimes used in production of chlorine dioxide.
HClO2 can be prepared through the reaction of lead chloride and dilute sulphuric acid.
Ba(ClO2)2+H2SO4→BaSO4+2HClO2
Pb(ClO2)2+H2SO4→PbSO4+2HClO2
Chlorous acid is a very powerful oxidizing agent, although its tendency to disproportionation counteracts its oxidizing potential.
Additional Information:
Chlorine is the only halogen to form an isolable acid of formula HXO2 . Neither bromous acid nor iodous acid has ever been isolated.
A few salts of bromous acid, bromites, are known, but no iodites.
Uses:
The use of chlorous acid is very limited due to its instability. However, its conjugate base chlorite is used as a salt of sodium that is used in some industrial processes as the production of chlorine dioxide.
The physical properties of chlorous acid have not been correctly determined due to its high instability. It has 1.96pKa and it is determined as the stability of the conjugate base of a determined acid. A value of 1.96 means the conjugate base chlorite ClO2 is stable.
Note:
As many compounds that contain chlorine, chlorous acid is extremely harmful if swallowed or inhaled. It can cause severe damages in eyes, mucous and skin. It is a strong oxidizing agent, however its reactivity is limited by its poor stability.
