Question
Question: Write the structure of A, B, C and D. 
Solution
The mixture of sodium nitrate and hydrochloric acid is going to be used to prepare diazonium chloride from aniline. By using diazonium chloride we can prepare halo benzene and benzene with different chemical reagents.
Complete answer:
- In the question it is asked to find the products A, B, C and D in the given chemical reactions.
- Coming to the given chemical reactions.
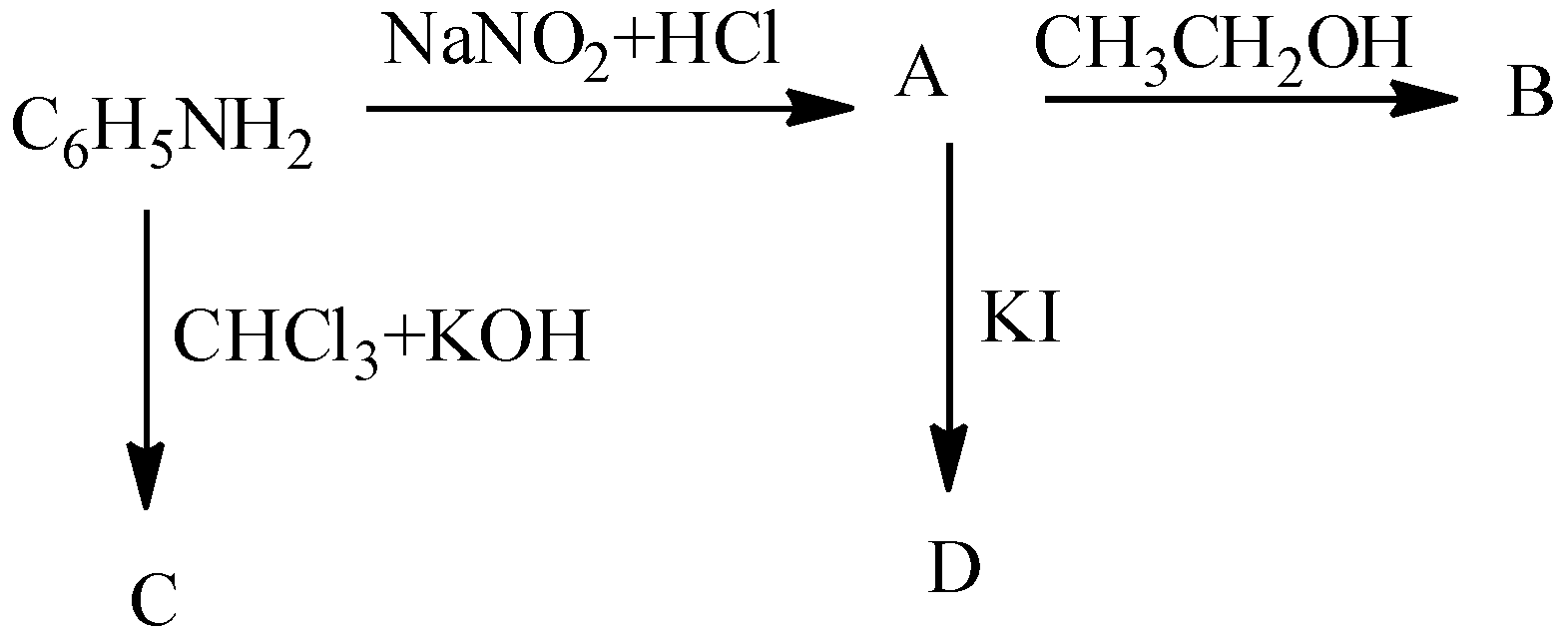
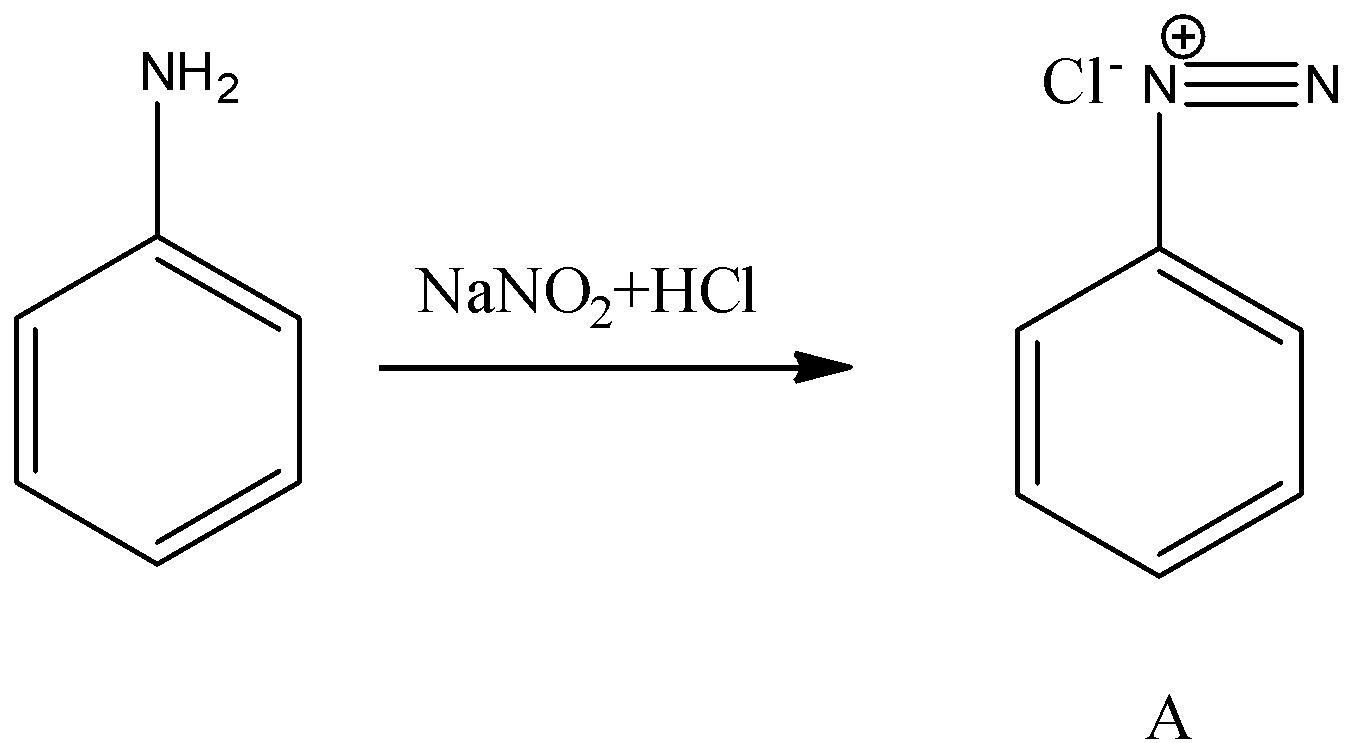
- First we will start with product A.
- We know that aniline undergoes diazotization and forms a chemical (A) called benzene diazonium chloride. The chemical reaction of converting aniline to benzene diazonium chloride (A) is as follows.
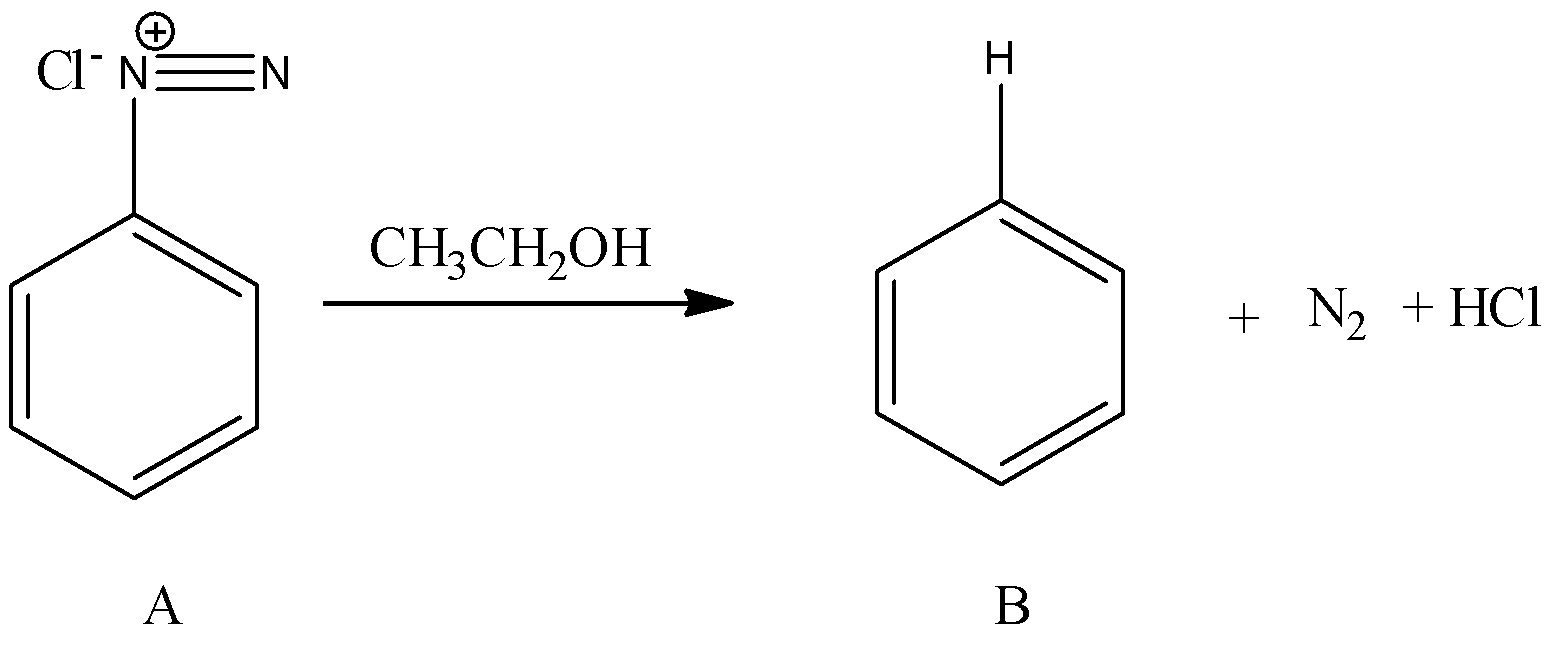
- Coming to the preparation B from A.
- The formed benzene diazonium chloride is going to react with ethanol and forms benzene as the product (B). The chemical reaction of converting A to B is as follows.
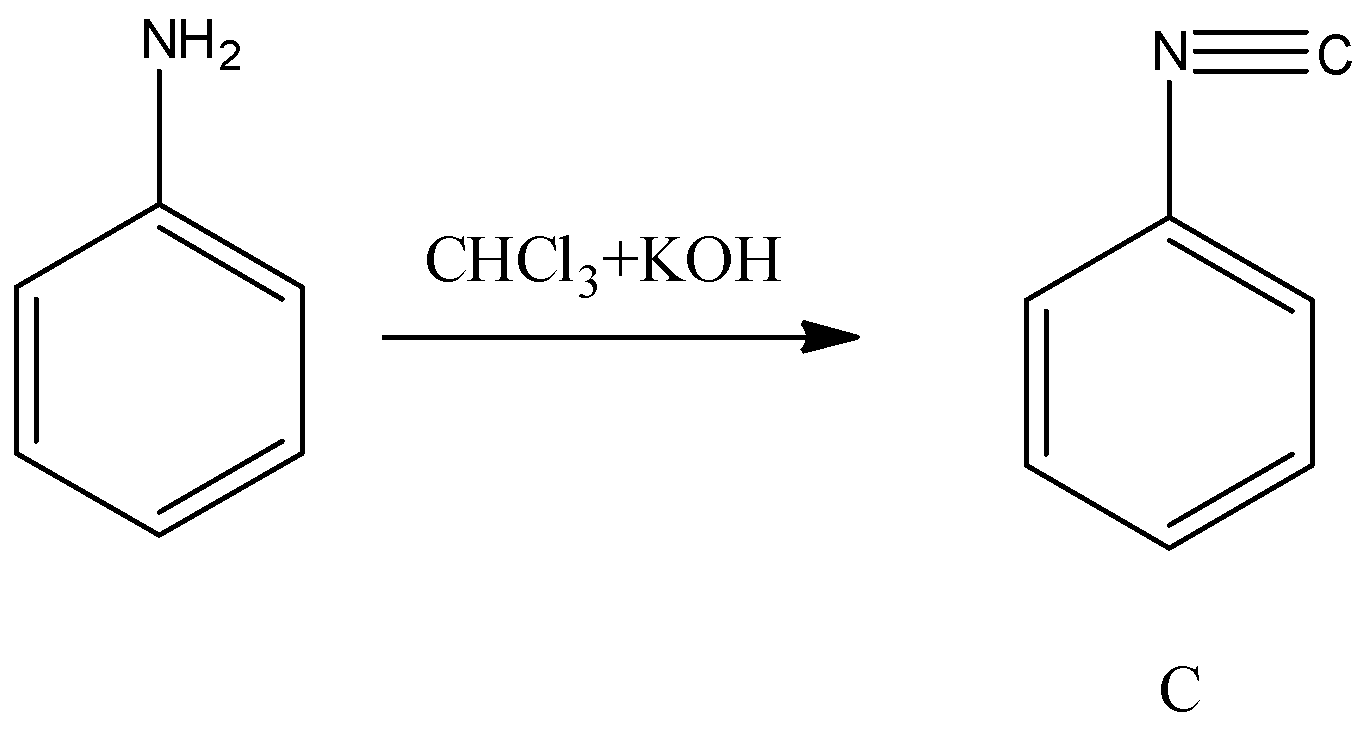
- Now coming to the preparation C from aniline.
- When aniline is going to react with chloroform and potassium hydroxide there is a formation of isocyanide compound (C). The chemical reaction of converting aniline to product C is as follows.
- Now coming to the preparation of from A, from A we can prepare iodo benzene by using potassium iodide. The chemical reaction of converting A to D is as follows.
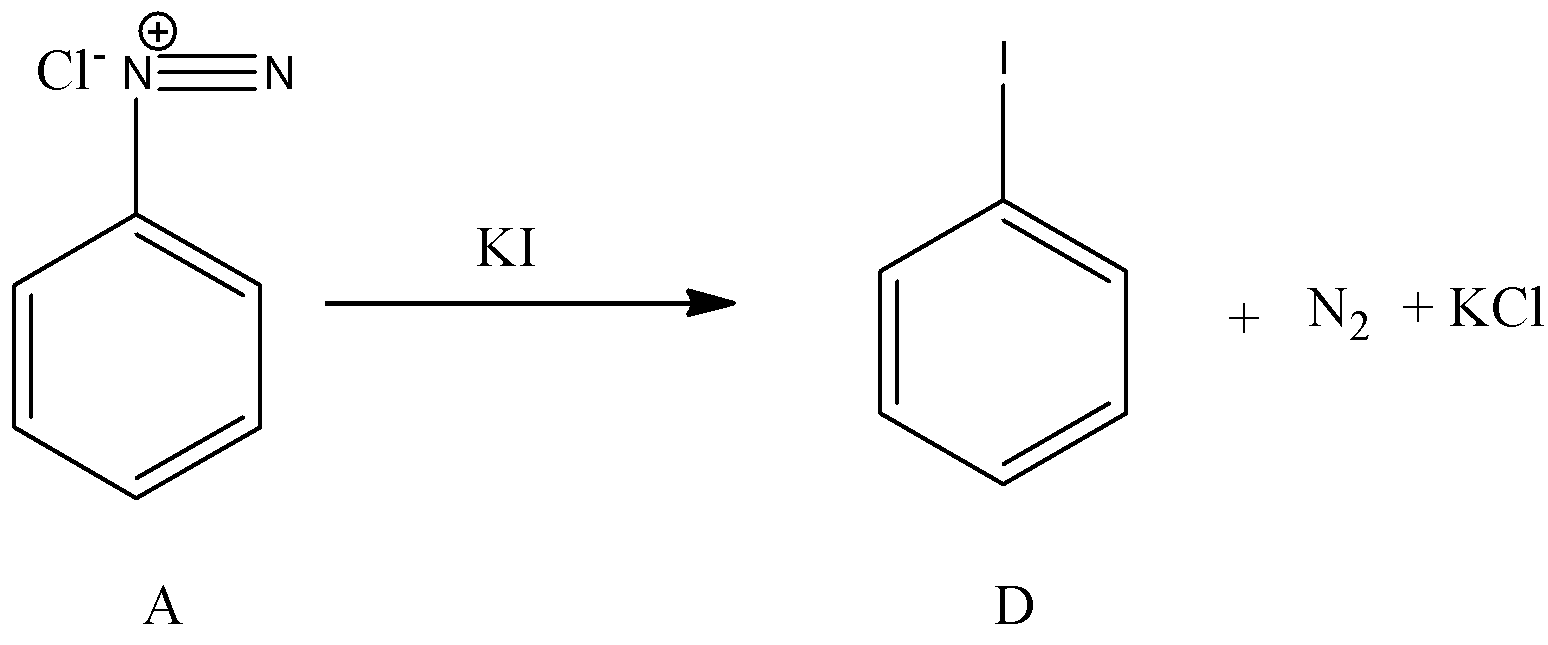
- Therefore the overall given chemical reaction is as follows.
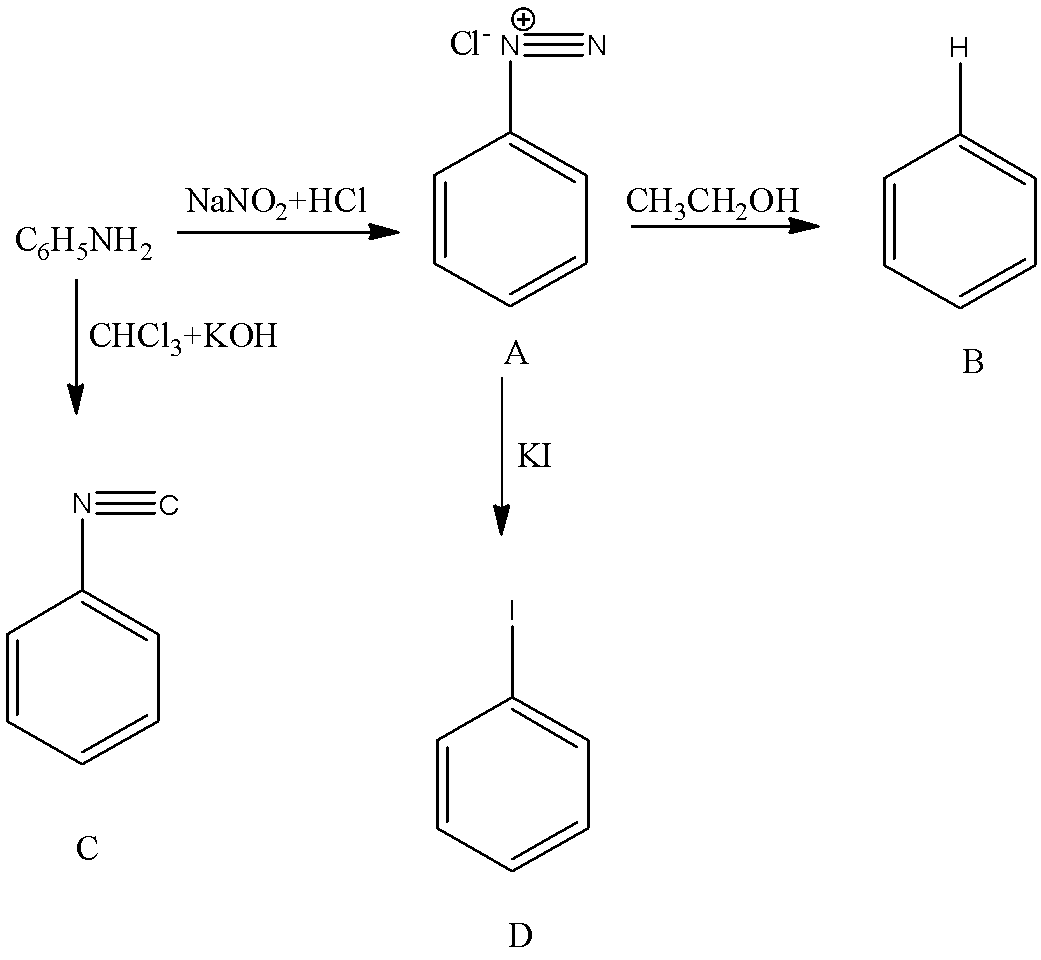
Note:
By using benzene diazonium chloride we can prepare a lot of different organic which have various applications in industries. The products we can prepare from diazonium chloride are iodo benzene, benzene, benzene isocyanide.
