Question
Question: Write the structural formulae of the following: Benzene b) cyclopropane c) Ethene...
Write the structural formulae of the following:
Benzene b) cyclopropane c) Ethene
Solution
Basically, we can identify the compounds by their molecular names. But these molecular formulas do not communicate about, by what means the elements are attached to each other. So, this is the place where the structural formula arises.
Complete step by step answer:
As we know that we can easily identify the compounds by their molecular formula. Though molecular formula demonstrates about the number of atoms but it does not tell us about the arrangement of atoms. So, this is the place where the structural formula arises. It displays how the atoms are organized and attached together in a molecular formula of a chemical compound.
Now, let’s talk about the first compound i.e. benzene. It is one of the most important organic compounds with chemical formula C6H6. It is a naturally occurring substance produced by volcanoes and forest fires and is present in many plants and animals as well. Moreover, it is also a major industrial chemical made from coal and oil. It is a six-carbon ring which includes three double bonds and each of the carbons represented by a corner is also bonded to one other atom. Its structure is as shown:
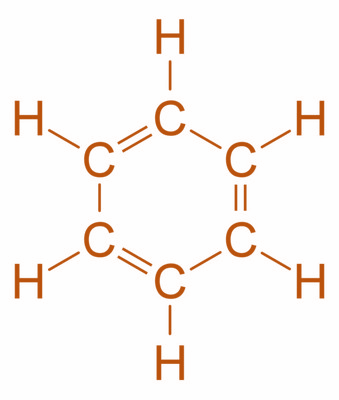
Now, the next one is cyclopropane. It is a cycloalkane with the molecular formula C3H6. It consists of three carbon atoms linked to each other to form a ring with each carbon atom bearing two hydrogen atoms. Its structure is as shown:
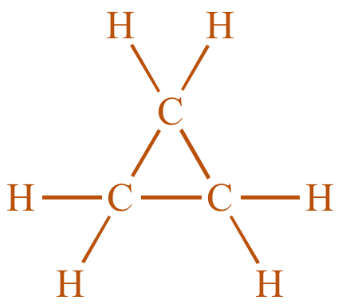
The last one is Ethene. It is a hydrocarbon which has the formula C2H4. It is a colorless flammable gas with a faint ‘sweet and musky’ odor. It is considered as the simplest alkene and is widely used in the chemical industry. Its structure is as shown:
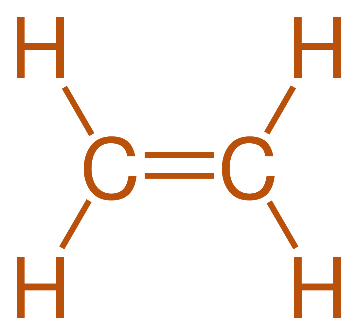
Note: The electron dot structural formula depiction uses dots to signify the electrons involved with the bonding of different atoms. Moreover, the line bond structural formula makes use of lines and bonds to show the covalent bonds between the atoms.
