Question
Question: Write the structural formula of the following: Isopropyl bromide...
Write the structural formula of the following: Isopropyl bromide
Solution
We need to know what is a structural formula. Organic molecules are denoted by a number of different notations. While a molecular formula only indicates the shapes and quantities of elements contained in a molecule, an extended structural notation structure may be used to define almost all of an organic material's compositional characteristics. The elemental symbols shown in the accompanying structural representation indicate the different sorts of elements that are present.
Complete answer:
We need to know that the chemical compound's structural formula is a graphical depiction of the molecular structure (derived using structural chemistry methods) that shows how the atoms could be organised in real three-dimensional space. Chemical connection inside the molecule is also demonstrated, either directly or implicitly. Unlike chemical formulae, which have a limited amount of symbols and descriptive capability, structural formulas give a more comprehensive geometric depiction of the molecular structure.
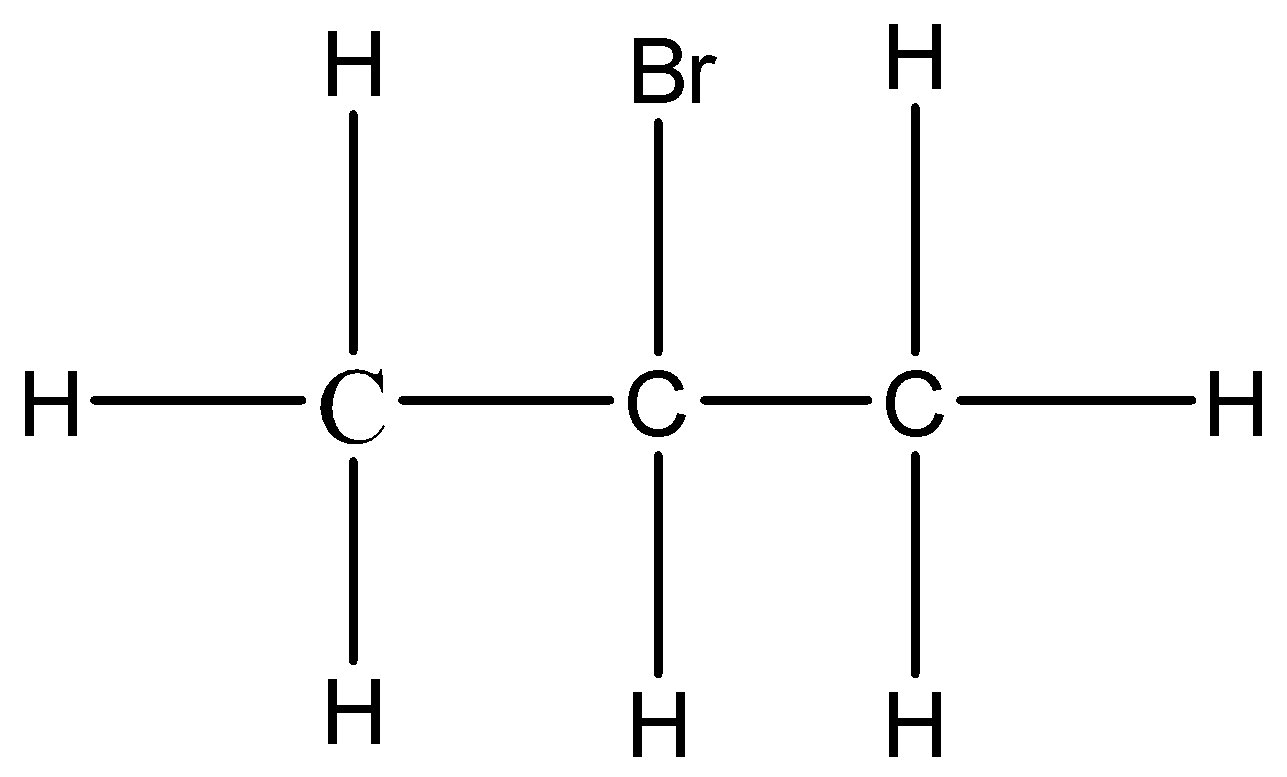
The given compound is Isopropyl bromide. The halogenated hydrocarbon with the formula CH3CHBrCH3 is 2-bromopropane, commonly known as isopropyl bromide and 2-propyl bromide.
Note:
We must note that isopropyl bromide is a clear liquid with no colour. It is used in organic synthesis to introduce the isopropyl functional group. Isopropanol is heated with hydrobromic acid to produce 2-bromopropane. Because the bromine atom is in the secondary position, the molecule may readily undergo dehydrohalogenation to generate propene, which escapes as a gas and can burst closed reaction vessels. When using this reagent in base catalysed processes, potassium carbonate should be substituted for sodium or potassium hydroxide.
