Question
Question: Write the structural formula of propyne....
Write the structural formula of propyne.
Solution
In organic chemistry, Propyne is an alkyne (methylacetylene). Along with its isomer propadiene (allene), which has been widely used in gas welding, it is a part of MAPD gas. Propyne can be safely concentrated, unlike acetylene. A chemical compound's structural formula is a graphic representation of the molecular structure, showing how the atoms may be organised in real, three-dimensional space. The chemical bonding within the molecule, either directly or implicitly, is also seen.
Complete step by step answer:
As we know that the position of chemical bonds between a molecule's atoms is defined by structural formulas. A structural formula consists of atom symbols linked by short lines representing chemical bonds; one, two, or three lines representing single, double, or triple bonds, respectively.
A chemical formula uses chemical element symbols, numbers, and sometimes other symbols, such as parentheses, dashes, brackets, commas, and plus, and minus signs, to present details about the chemical proportions of atoms that constitute a particular chemical compound or molecule
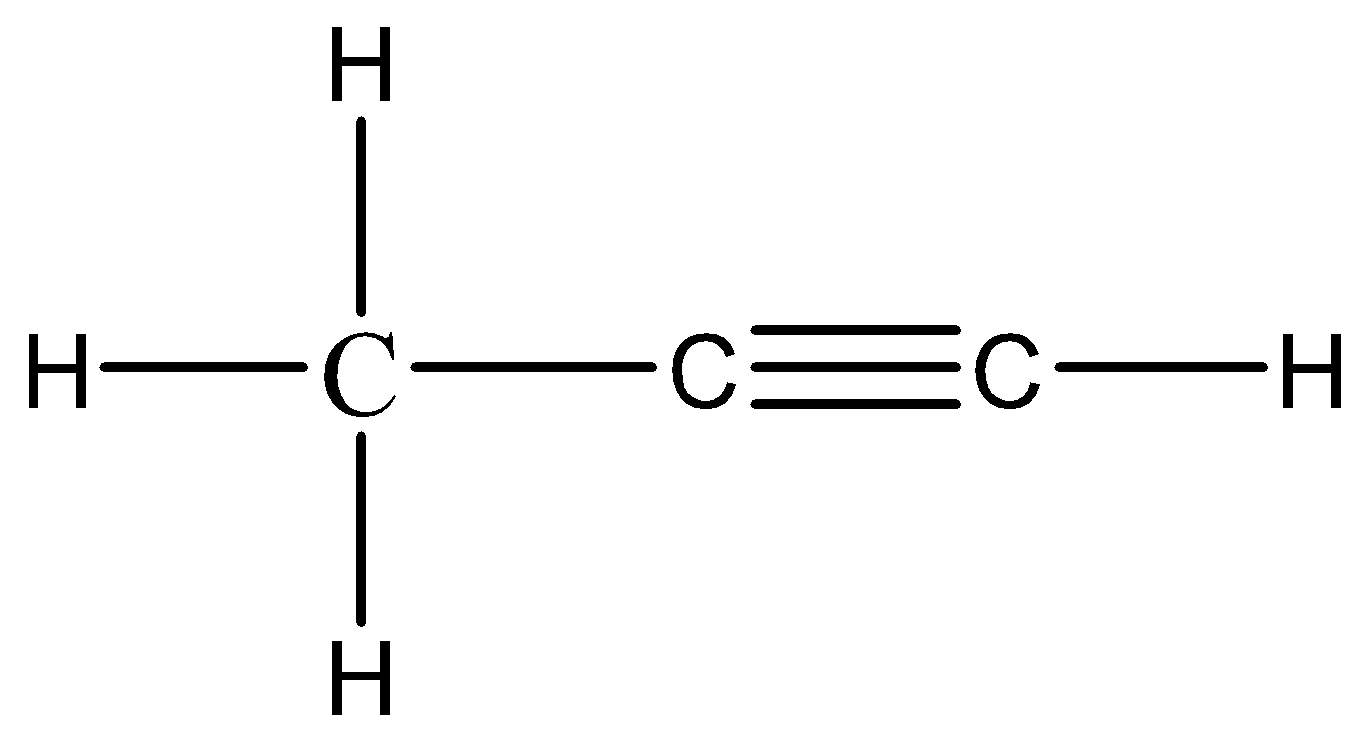
The formula of propyne is C3H4
The structural formula is:
Note: We must have to remember that the propyne for organic synthesis is a convenient three-carbon construction block. N-butyllithium deprotonation yields propynyllithium. This nucleophilic reagent adds alcohol and ester production to the carbonyl groups. The second simplest member of the acetylene family is Propyne. It may form mixtures of explosives with oxidising agents and air. It is used as fuel for welding torches. Propyne for organic synthesis is a convenient three-carbon construction block. N-butyllithium deprotonation yields propynyllithium. This nucleophilic reagent adds alcohol and ester production to the carbonyl groups. MAPP gas may be used to produce large quantities of the reagent cheaply, though purified propyne is costly.
