Question
Question: Write the structural formula of ethene (Ethylene)?...
Write the structural formula of ethene (Ethylene)?
Solution
To answer this question we should be aware of alkene. Ethylene is widely used as a plant growth regulator that helps the process of senescence, ripening, and abscission in plants. It is marketed under the trade name serafin.
Complete Solution :
- Alkenes are the hydrocarbons with general formula CnH2n where n is the number of carbon atoms. It is an unsaturated hydrocarbon as alkene contains c-c double bond in their molecules. Alkenes also known as Olefins where, Oleum means oil and fines means to care. The lower members of alkene form oily products on treating it with bromine or chlorine. They are produced by cracking of petroleum.
- The first member of the alkene series is ethylene, the number of carbon atoms in ethylene is 2.
General formula = CnH2n
n = 2 ( for ethylene)
Substituting n in general formula,
C2H4
- The chemical formula of ethylene is C2H4.
- In ethylene the carbon atoms are of sp2 hybridized . They are attached to each other by one σ bond and a σ bond. The σ bond is obtained by the overlap of sp2 hybrid orbital and the π bond is obtained by the overlap of unhybridized p orbitals.
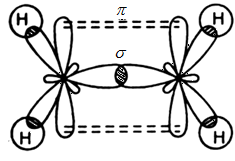
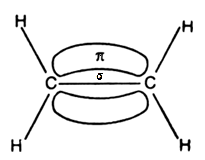
These two above mentioned diagrams are orbital structures of ethylene.
- The structural formula is:
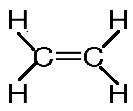
Note: The bond angle ∠HCC and ∠HCH are 120∘. The bond angle of c = c is 1.34 A∘. In ethylene the carbon is sp2 hybridized. In case of alkane the carbon will be sp3 and in case of alkyne the carbon is sp hybridized. Ethylene and ethene are the same. Ethene is the IUPAC name and ethylene is a common name.
