Question
Question: Write the role of dilute \[{\rm{NaCN}}\] in the extraction of gold....
Write the role of dilute NaCN in the extraction of gold.
Solution
We know that extraction is a process in which the impure ore can be treated in several processes for getting pure metals. The process of removal or separation of impurity from the ore is termed as the extraction process.
Complete answer
As we All know, gold extraction is that process which is usually required for the separation of gold from its ore. The process of extraction involves mineral processing, pyro metallurgical process and hydrometallurgical process.
The extraction of gold ore involves several steps. The first step used for the extraction of gold is crushing girding. In this step, we have to make a small particle of the original ore by using a crusher or grinder. In the second step, gravity separation or flotation takes place. In this process the particles which are dissolved, they generally float on the upper surface. In the third step, leaching. In this step the ore is extracted from the hydrometallurgical process it converts the gold from low-grade ore to the old which is soluble in water and forms coordination complexes. The fourth step is refining. In this step, we get refined metal which does not contain any impure substance in it. The diagram is shown below.
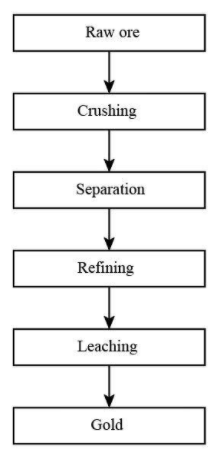
So, in the extraction process of gold, dilute NaCN can be used as a leaching agent. The gold is generally converted into a cyanide complex from which the metal is separated. The leaching of gold is involved in the presence of air. The reaction is shown below.
4Au(s)+8NaCN(aq)+H2O+O2→4Na[Au(CN)2]+4KOH
Note:
The application of ore extraction are agricultural industry, food industry, pharmaceutical and used for purification of base metals and used for refining one of the precious metals.
