Question
Question: Write the resonating structures for \(CO_3^{2 - }\) and \(HCO_3^ - \) ions....
Write the resonating structures for CO32− and HCO3− ions.
Solution
Whenever for a molecule we can write two or more than two lewis structure but none of them is able to explain all properties of the molecule but collectively they describe all properties of that molecule is known as the phenomena of resonance and all the structures are known as resonance hybrid or conical structure.
Complete step by step answer:
There are some rules for writing the resonating structures which are discussed below:
1.The various resonating structure should differ only in the position of electrons and not in the position of atoms
2.All the resonating structures should have the same number of unpaired electrons.
3.All the resonating structure should have similar energy
The resonating structure CO32− is given as:
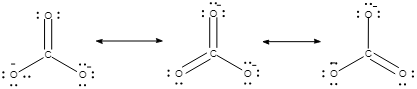
And the structure of HCO3− ion is given as:

Some application of resonance given below:
1.It helps to explain the ortho, para, meta directing influence of substitution in benzene.
2.It helps to explain the low reactivity of aryl and vinyl halides in nucleophilic reactions.
3.It helps to explain the acidic nature of phenols and carboxylic acid.
4.It also helps to explain the reactivity of allyl and benzyl halides.
Additional information:
Resonance measures the stability of the molecules. The greater the number of resonating structures, the more stability.
Resonance energy gives the measure of the stability of resonance hybrid, more will its value greater will be stability.
Note:
When two different resonating structures have the same number of bonds, the resonance is called isovalent resonance on the other hand if two different resonating structures have a different number of bonds then they are called heterovalent.
