Question
Question: Write the resonance structure that would result from moving the electrons as the curved arrows indic...
Write the resonance structure that would result from moving the electrons as the curved arrows indicate. Be sure to include formal charges if needed.
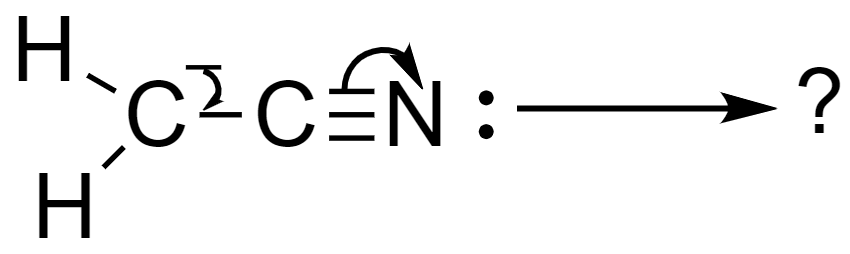
Solution
When an ion or an atom or a molecule shows resonance, the average of two or more resonance structures can be used to define the given compound. These resonance structures are separated from each other by double-headed arrows.
Complete answer:
The multiple resonance structures that depict the delocalization of electrons in a polyatomic ion or a molecule are known as resonance structures.
In chemistry, the bonding of atoms in particular ions and molecules can be depicted by combining various contributing forms as per the valence bond theory into hybrid structures or hybrid resonance.
These contributing forms are known as resonance structures or canonical structures.
In a resonance hybrid, the delocalization of electrons takes place via the formal charges or the fractional bonds.
The best resonance structure depicting the actual bonding of the molecule must be determined since not all resonance structures are the same.
Formal charges can be used to predict the resonance structures that are favored.
Now, the carbonation given to us is cyanomethane. In this anion, the presence of a triple bond between the carbon atom and the nitrogen atom (C≡N), and the presence of the negative formal charge on the carbon atom, the electrons, and the bonds undergo conjugation to form a new resonating structure, vinylidene amide.
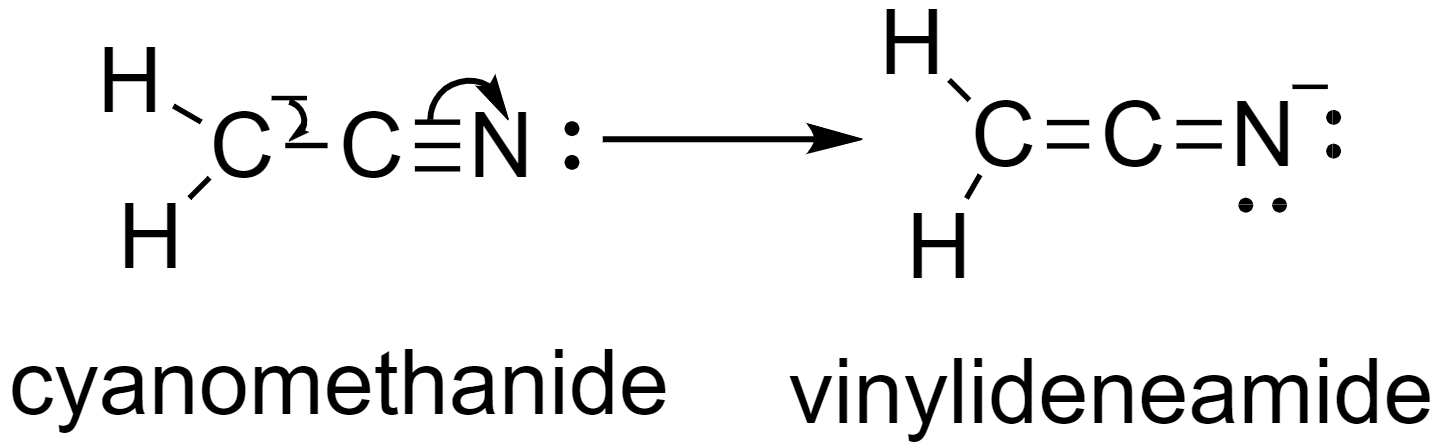
Here, the negative formal charge on the carbon atom moves to the bond between the two carbon atoms to form a double bond, and one of the bonds between the carbon and the nitrogen atoms moves to the nitrogen atom to form a negative formal charge.
Note:
We know that
Formal charge = valence electrons - (lone pair electrons + 21(bonded electrons))
So, the formal charge on nitrogen atom will be
Formal charge = 5 - (4 + 21×4)
Formal charge = -1
So, the formal charge on the nitrogen atom will be -1.
