Question
Question: Write the resonance structure of carbonate ion?...
Write the resonance structure of carbonate ion?
Solution
Draw the resonance structure by the delocalized double bond character of the carbonate ion. The carbonate ion is represented as CO32−
Complete step by step solution:
Resonance structures are shown by polyatomic molecules or ions in which sets of lewis structure shows delocalization of electrons. It is also made to describe the manner of bonding in a particular ion or molecule by merging many structures called resonance structures.
In this question, we have to describe the resonance structure of carbonate ions. Carbonate ion is a polyatomic ion commonly found in limestone.
Let us first arrange the carbon and oxygen atom in carbonate ion (CO32−),
-Among oxygen and carbon, carbon is the least electronegative, thus we put carbon in the central position and oxygen as a surrounding atom.
-The no of valence electrons in the outermost orbital of carbon is 4 and oxygen have 6 electrons in their outermost orbital and in carbonate, there are two more electrons as a charge so the no of valence electrons will be 24.
-Among them, six electrons are used to form the three bonding pairs between carbon and oxygen and the remaining electrons are divided among each oxygen atom equally as a lone pair and also indicating the -2 charge.
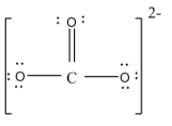
- but at this time the carbon atom will have only 6 valence electrons so to complete the eight electrons it will form a double bond with one of the oxygen atoms. Thus the structure will be,
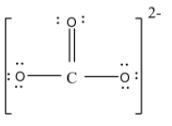
Here we can draw this structure in three possible ways by delocalizing the double bond and these structures are known as resonating structures of each other. The structures are,
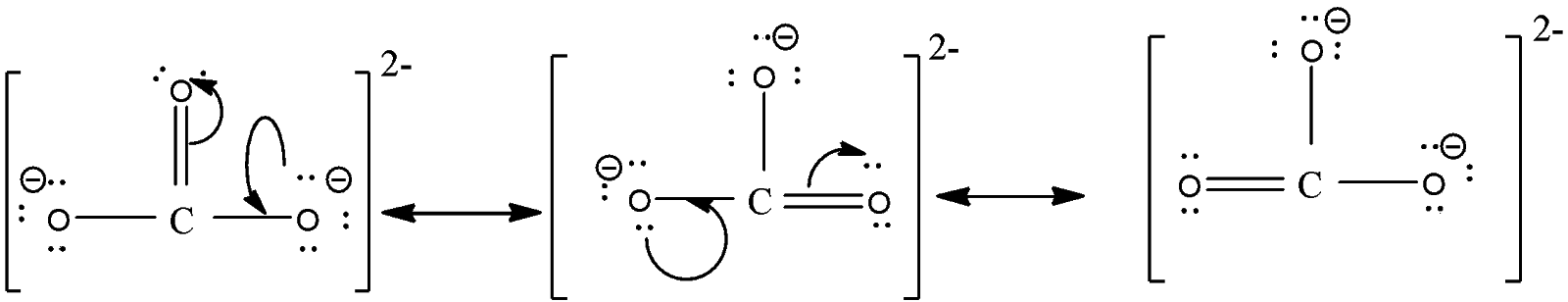
In the diagram, you can see that there are one carbon-oxygen double bond and two carbon-oxygen singles but the bond length of all C-O bonds will remain the same.
Note: While drawing the resonance structure, delocalize the double bond and do not change the structure of the compound. The bond length of both single and double bonds will remain the same. The charge should be taken into consideration and must be drawn in the structure.
