Question
Question: Write the reason for \(C{{O}_{2}}\) can be easily liquified and even solidified?...
Write the reason for CO2 can be easily liquified and even solidified?
Solution
Carbon dioxide is gas, having the molar mass as 44 and has very weak intermolecular forces and very low melting point as well as the boiling point. Now using these points you can easily explain the reason for solidification and liquefaction for carbon dioxide molecules.
Complete step by step answer:
First let's discuss carbon dioxide. Carbon dioxide is a gas which is formed by the reaction between the two non-metals i.e. carbon and oxygen. The chemical is supposed to take place as;-
C+O2→CO2
Now coming to the terms solidified and liquified. By the term solidification, we mean the conversion of the solid into the liquid and the term liquefaction means the conversion of liquid into gas.
Now considering the statement as;-
Carbon dioxide gas i.e. CO2 can be easily liquified and even solidified due to the following reasons as:-
- Intermolecular force of attraction:- The intermolecular forces of attraction between the carbon dioxide molecules is very low and hence can be easily liquified and as well as solidified.
- Melting point:- The melting point of the carbon dioxide molecule is very low i.e.−56.6∘C - hence can be easily liquified.
- Boiling point:- The boiling point of the carbon dioxide molecule is very low i.e.30.98∘C - hence can be easily solidified.
Thus, these reasons clearly explain that CO2 can be easily liquified and even solidified.
Note: The structure of the carbon dioxide molecule is as;-
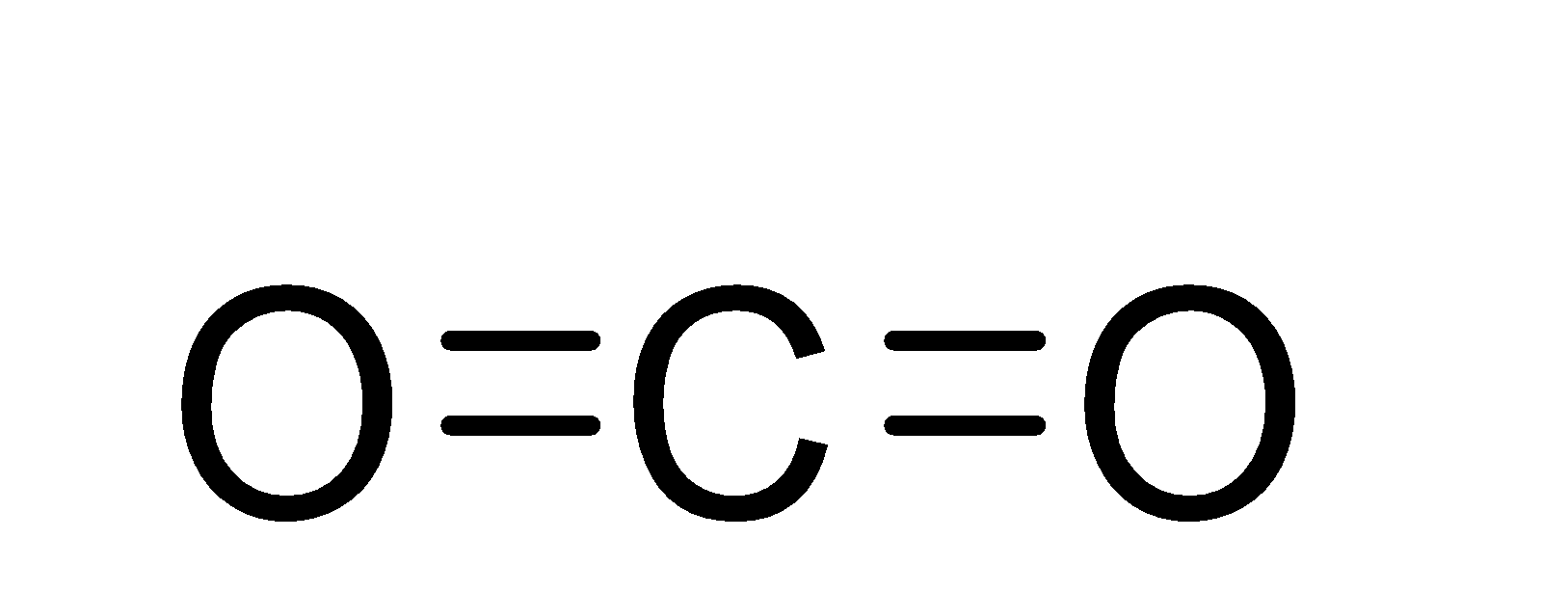
From the structure, we can see that there are a total number of four bonds in the carbon dioxide molecule and out of these 4 bonds, 2 are sigma bonds and two are pi-bonds.
