Question
Question: Write the products in the given reaction: i. \[C{{H}_{3}}CH=CHCN\xrightarrow[{{H}_{2}}O]{DIBAL-H}\...
Write the products in the given reaction:
i. CH3CH=CHCNDIBAL−HH2O
ii. 
iii. 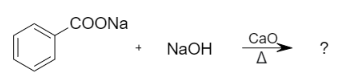
Solution
A chemical reaction is defined as a process in which the bond of the reactant molecules breaks and results in the formation of a new bond that gives a product molecule. It is a reaction in which two or more molecules interact and form new substances.
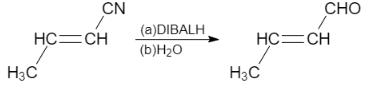
Complete step-by-step answer: i. DIBAL−H is a organoaluminum compound which act as a reducing agent. When but−2−enenitrile reacts with DIBAL−H, it results in the formation of but−2−enal. In this reaction, the cyano group gets reduced to aldehyde group and the carbon- carbon double bond remains intact.
Let us see the reaction:
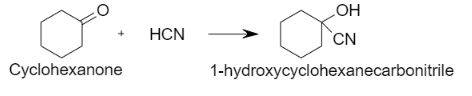
ii. Hydrogen cyanide when added to aldehydes and ketones, it results in the formation of hydroxybutanenitrile adducts which are mainly known as cyanohydrins. When Cyclohexanone reacts with hydrogen cyanide, it results in the formation of hydroxy cyclohexanecarbonitrile. In this reaction, hydrogen cyanide is added to the carbonyl bond.
Let us see the reaction:
iii. Decarboxylation reaction is defined as the reaction in which a carboxyl group is removed and releases carbon dioxide. It is a reaction of carboxylic acids that removes a carbon atom from a carbon chain. When sodium benzoate is heated with sodium hydroxide and calcium oxide, it results in the formation of benzene.
Let us see the reaction:
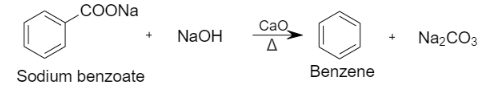
Note: In this question we concluded that a chemical reaction takes place when a substance is converted into another substance. The mixture of sodium hydroxide and calcium oxide is known as soda lime. In a chemical reaction, soda lime acts as sodium hydroxide but it is not deliquescent.
In chemistry, every compound has a molecular formula on the basis of which a structure of compound is formed. Chemical formula is used to represent any chemical substance with the help of symbols of atoms present in it. Students get confused while writing the name of a chemical compound in a reaction and therefore it is necessary to have detailed knowledge of the formation of compounds.
