Question
Question: Write the preparation of benzoic acid from the following: (a) Styrene (b) Benzamide (c) Dry ic...
Write the preparation of benzoic acid from the following:
(a) Styrene
(b) Benzamide
(c) Dry ice
Solution
To solve this question, we first need to know what benzoic acid, styrene, benzamide, and dry ice.
Benzoic acid: A colorless and crystalline derivative of benzene having formula C6H5COOH
Styrene: A colorless and oily derivative of benzene having formula C6H5CH=CH2.
Benzamide: A white and solid derivative of benzoic acid having formula C6H5C(O)NH2.
Dry ice: A cooling agent which is the solid form of the gas carbon dioxide CO2.
Complete answer:
Let us look at the reactions involving the preparation of benzoic acid from styrene, benzamide, and dry ice.
a) Using styrene (phenylethane) to prepare benzoic acid.
While converting styrene to benzoic acid, an oxidizing agent is used.
Either potassium permanganate or potassium dichromate, in the presence of sulfuric acid, acts as a strong oxidizing agent and converts styrene to benzoic acid.
Styrene is first converted to potassium benzoate and then acidified to give benzoic acid.
The reaction proceeds as follows:
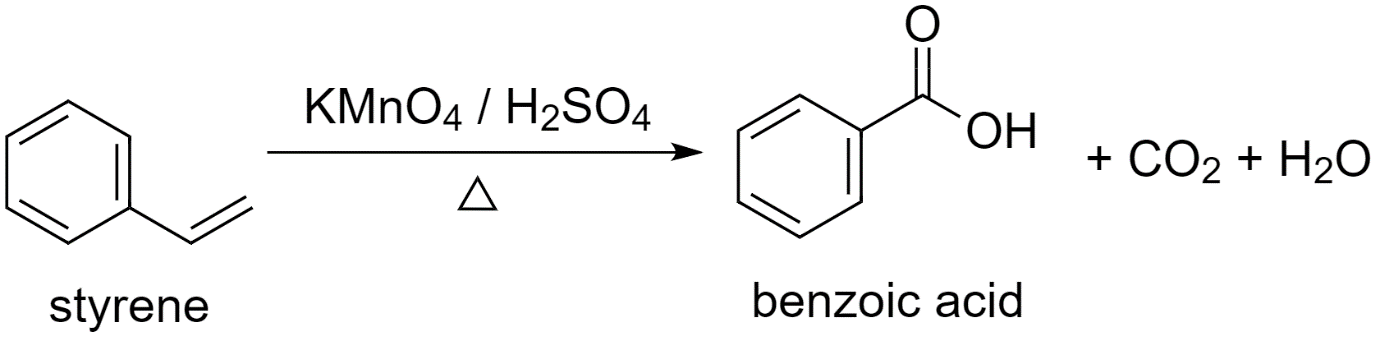
Along with benzoic acid, carbon dioxide CO2 gas is released and a water H2O molecule is produced as by-products.
b) Using benzamide to prepare benzoic acid.
In the presence of an acid like dilute hydrochloric acid, the hydrolysis of benzamide with sodium hydroxide (NaOH) forms benzoic acid.
Benzamide is first converted to sodium benzoate which is further acidified to benzoic acid.
The reaction proceeds as follows:
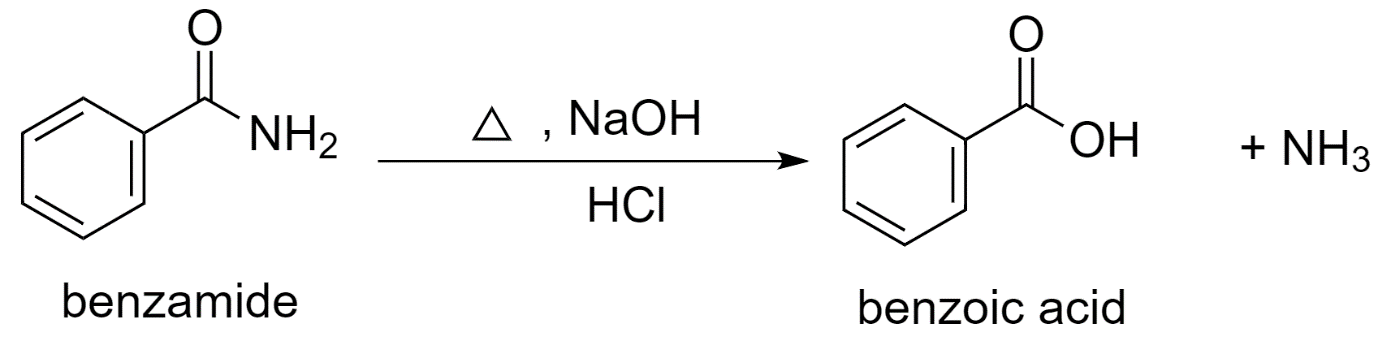
Along with benzoic acid, ammonia NH3 is released as the by-product.
c) Using dry ice to prepare benzoic acid.
When dry ice or solid carbon dioxide CO2 is added to a solution of phenyl magnesium iodide in dry ether, an adduct is formed. The adduct formed is a magnesium salt of carboxylic acid.
When this adduct is hydrolyzed in the presence of an acid, it gives benzoic acid.
The reaction proceeds as follows:
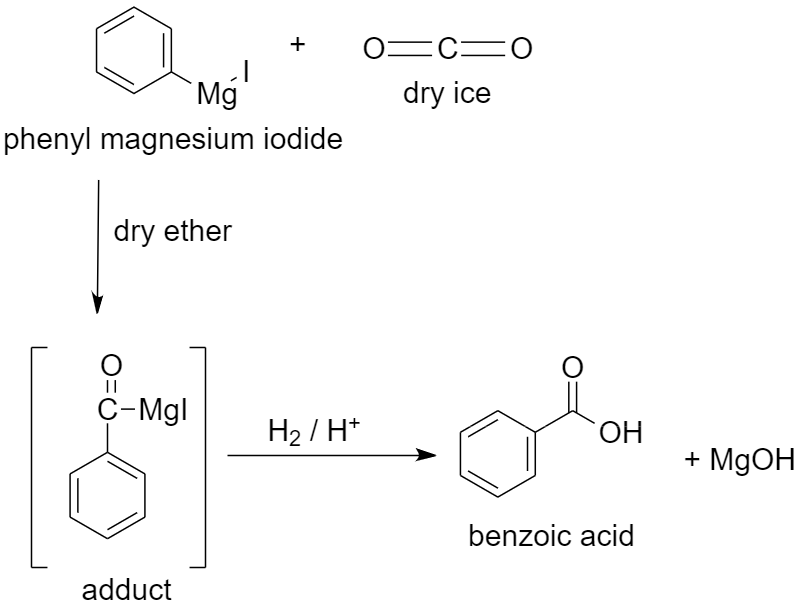
Along with benzoic acid, magnesium hydroxide (MgOH) is formed as a by-product.
Note:
It should be noted that the gas released from dry ice can increase the levels of carbon dioxide CO2 in the blood and can cause hypercapnia.
Also, styrene is considered to be carcinogenic and toxic, especially when in contact with skin or eyes.
Benzoic acid on the other hand is produced naturally in many plants and animal species.
