Question
Question: Write the number of covalent bonds in the molecule of butane, \[{C_4}{H_{10}}\]....
Write the number of covalent bonds in the molecule of butane, C4H10.
Solution
Molecules consist of tiny particles called atoms. Atoms in molecules are attached to one another by chemical bonds. The chemical bonds were of different types. The bond formed by sharing one electron from each atom can be called a covalent bond. Covalent bond is formed between atoms with similar electronegativities.
Complete answer:
Alkanes are also known as paraffins. The hydrocarbons are the molecules which consist of carbon and hydrogen atoms and can be called hydrocarbons. Thus, alkenes also belong to hydrocarbons. The general molecular formula of alkanes is CnH2n+2, where n is the number of carbon atoms.
Here the given compound is butane with the molecular formula of C4H10.
All atoms are bonded to another atom through single bonds only as alkanes are saturated compounds. The electronegativity is not having much difference between carbon and hydrogen. Thus, covalent bonds will be formed.
The structure of butane will be
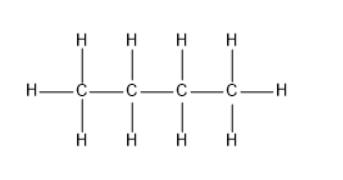
The straight lines in the above structure is a covalent bond. There are a total of 13 straight lines in the above structure. Thus, there are 13 covalent bonds in butane.
Note:
Chemical bonds formed between the atoms by transfer of two electrons from the same atom to another atom can be called ionic bonding. When each atom contributes one electron, the sharing of two electrons between two atoms leads to the formation of covalent bonds. Electronegativity plays an important role in bonding.
