Question
Question: Write the names and structures of the monomers of the following polymers: (i) Nylon-6,6 (ii) PHB...
Write the names and structures of the monomers of the following polymers:
(i) Nylon-6,6
(ii) PHBV
(iii) Neoprene
Solution
Polymer is a substance made of a large number of smaller units or molecules which link to each other to form larger molecules. Those smaller units or molecules are referred to as monomeric units. Polymer can occur naturally or we can manufacture them synthetically.
Complete step by step solution:
Polymers are the substances made of long, repeating chains of small units or molecules (called monomers). These materials have unique properties, depending on the type of molecules being bonded. Polymers can be classified as addition polymer (formed by the addition of monomers without elimination of anything) and condensation polymer (formed by the combination of monomers with the elimination of simple molecules like water, etc.).
Let us see the names and structures of monomers of the given polymers:
(i) Hexamethylene diamine reacts with adipic acid and forms Nylon-6,6. It is a condensation polymer formed by combination of these two components (monomers) with loss of water molecule. So, the monomeric units of this polymer are hexamethylenediamine and adipic acid. Their structures are as follows:
Structure of hexamethylenediamine:
H2N−CH2−CH2−CH2−CH2−CH2−CH2−NH2
Structure of adipic acid:
HOOC−CH2−CH2−CH2−CH2−COOH
(ii) Poly(3−hydroxybutyrate-co-3−hydroxyvalerate), commonly called as PHBV is a copolymer of 3−hydroxybutanoic acid and 3−hydroxypentanoic acid in which the two monomer units are connected by ester linkages. Hence, the name of the monomeric unit of this polymer are 3−hydroxybutanoic acid and 3−hydroxypentanoic acid. Their structures are:
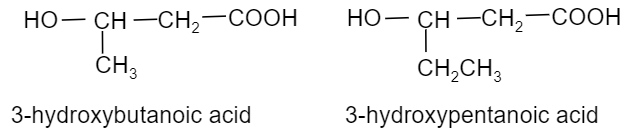
(iii) Neoprene is a synthetic rubber. It is a polymer of 2−chloro−1,3−butadiene or chloroprene and so it is also called polychloroprene. The chloroprene needed for this is prepared by the addition of hydrochloric acid or vinylacetylene. So, the monomer of this polymer is chloroprene and its structure is as follows:
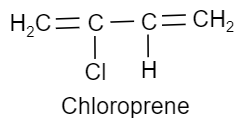
Note: Neoprene serves as an excellent rubber like material. It is used as an insulator and for making conveyor belts. PHBV is used in orthopaedic devices and in controlled drug release. Nylon-6,6 is widely used as fibres.
