Question
Question: Write the names and structures of the monomers of the following polymers. A) Bakelite B) Nylon-6...
Write the names and structures of the monomers of the following polymers.
A) Bakelite
B) Nylon-6,6
C) Polythene
Solution
A molecule that can react with other molecules to form very large molecules having high molecular weights is known as a monomer. The high molecular weight molecules formed are known as polymers. The monomer is a repeating unit of polymer.
Complete step by step answer:
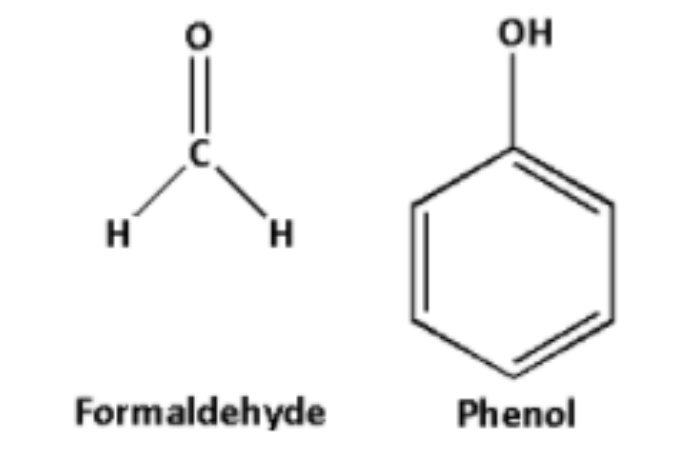
A) Bakelite is a polymer formed by the polymerization reaction of phenol and formaldehyde.
Thus, the monomers of bakelite are phenol and formaldehyde.
The structures of phenol and formaldehyde are as follows:
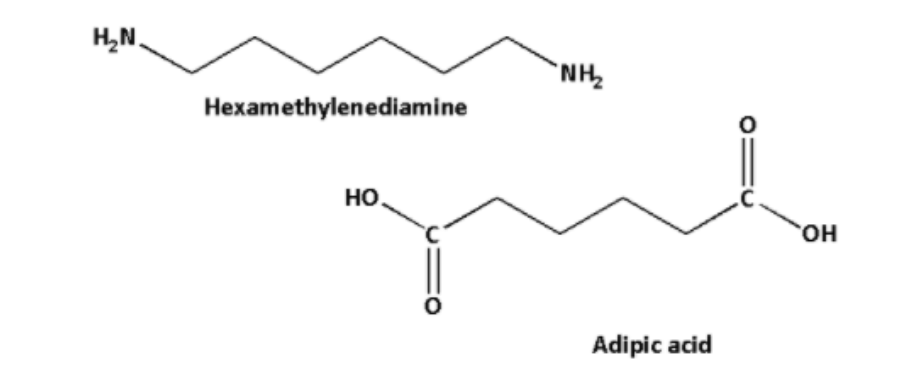
B) Nylon-6,6 is a polymer formed by the polymerization reaction of hexamethylenediamine and adipic acid.
Thus, the monomers of nylon-6,6 are of hexamethylenediamine and adipic acid.
The structures of of hexamethylenediamine and adipic acid are as follows:
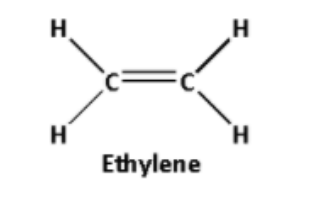
C) Polythene is a polymer formed by the polymerization reaction of ethylene molecules.
Thus, the monomer of polythene is ethylene.
The structure of ethylene is as follows:
Note: Bakelite is manufactured in the form of sheet, rod and tube. It has many industrial applications in electronics, power generation and aerospace industries. The colour of Bakelite is usually brown but it can be manufactured in many bright colours.
- Nylon-6,6 is very light in weight. Thus, it is used in the manufacturing of parachutes. It is waterproof in nature and thus is used in making swimwear. Other uses of nylon-6,6 are manufacturing of fishnets, airbags, tents, machine parts, etc.
- Polythene is generally used as a packaging material. Plastic bags, plastic films, bottles, and geomembranes are made from polythene. Polythene is non-biodegradable and its increasing accumulation in the atmosphere is a threat to nature.
