Question
Question: Write the name and structure of any five oxoacids of phosphorus?...
Write the name and structure of any five oxoacids of phosphorus?
Solution
. We know that oxoacids contain oxygen. In the oxoacids, there are different types of bond-like P-OH, P=O bond, and many more. The formation of different oxoacids also depends upon the oxidation state of phosphorus. We can name, and draw the structure of phosphorus.
Complete step by step answer:
-First, we will discuss the bonds found in the oxoacids; as mentioned there is the presence of one P−OH,or P=O bond. But bonds like P-P or P-H can be also there in consideration with the other bonds.
-We also mentioned the factor oxidation state, then it should be less than +5 for the phosphorus.
-Now, we will draw the structures of five oxoacids of phosphorus.
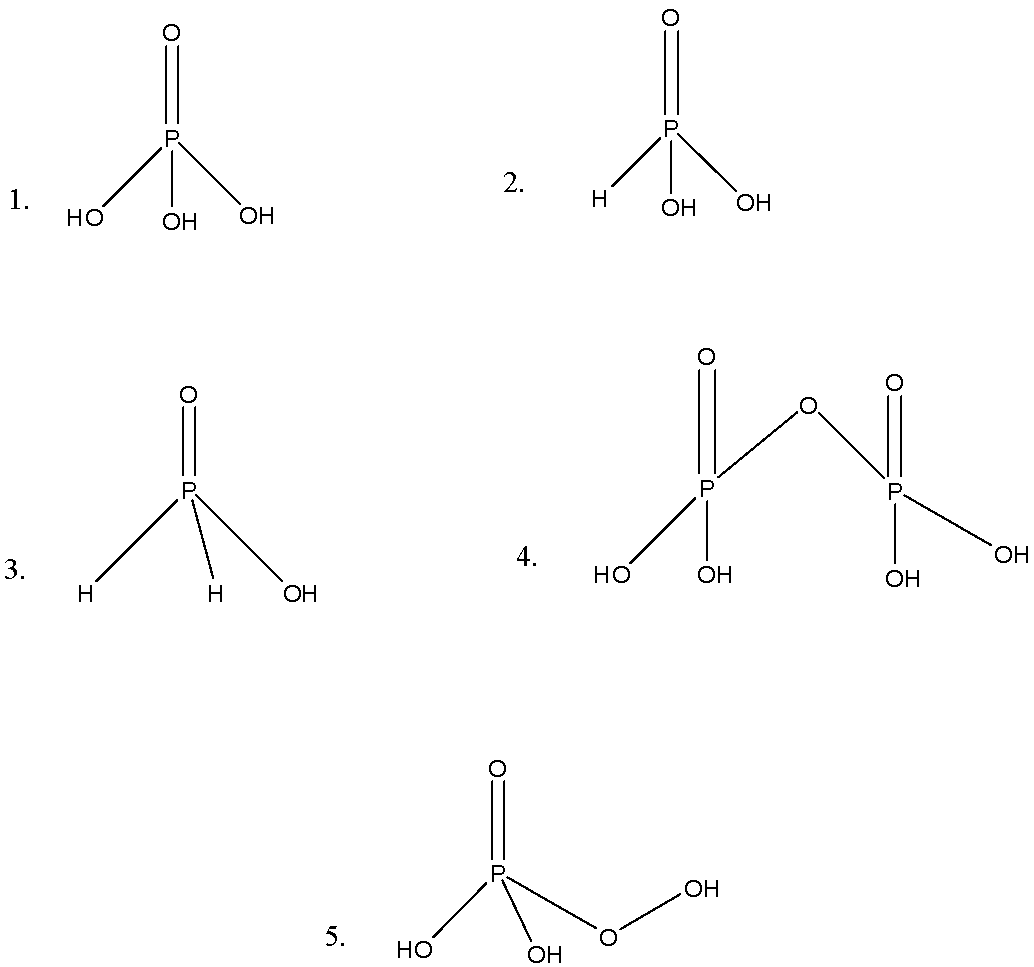
-Now, we can see the five structures. Let us discuss these structures.
-The first we have H3PO4, in this structure, there are three P-OH bonds and one P=O bond; it represents the tetrahedral representation. It is named as orthophosphoric acid.
-The second structure we have H3PO3, in this structure we can see P-H bond in addition to the P-OH, and P=O bonds. It is named as orthophosphorous acid.
-The third we have H3PO2, in this structure we can see the presence of 2P-H bonds, and it is named as hypophosphorous acid.
-The fourth we have H4P2O7, in this we can see the presence of P-O-P bond, and it is named as pyrophosphoric acid.
-The last we have H3PO5, in this structure we can see the P-O-OH bond, or we can say peroxide bond, so it named as polyphosphoric acid.
-In the last, we can conclude that the above mentioned are oxoacids of phosphorus.
Note: Don’t get confused while drawing the structures of the oxoacids of phosphorus, just complete the valency of phosphorus. In all these structures phosphorus depicts the oxidation state less than 5.
