Question
Question: Write the name and structure of an alcohol with four carbon atoms in its molecule....
Write the name and structure of an alcohol with four carbon atoms in its molecule.
Solution
Connect four carbon atoms in the chain and write the name of alcohol. General formula of alcohol is CnH2n+2O . Alcohols are having functional groups -OH. Alcohols can be classified into primary alcohol, secondary alcohol and tertiary alcohol.
Complete step by step answer:
Molecular formula of alcohol with four carbon atoms is C4H10O . Alcohol with four carbon atoms having more than one structural formulae which are known as isomers. Four constitutional Isomers of C4H10O with their IUPAC names and common names in brackets are following as:
1. Butan-1-ol (n-Butyl alcohol)
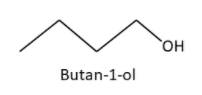
2. Butan-2-ol (sec-Butyl alcohol)
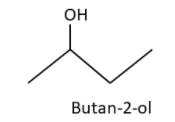
3. 2-Methylpropan-2-ol (tert-Butyl alcohol)
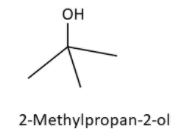
4. 2-Methylpropan-1-ol (Isobutyl alcohol)
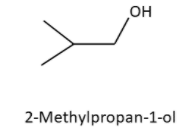
Butan-2-ol isomer is an asymmetric molecule and optically active. Butan-2-ol is having 2 enantiomers i.e. dextrorotatory and levorotatory isomers, which are optically active. Constitutional isomers of C4H10O are four and total isomers of C4H10O are five including stereoisomers. Molecule number 1 and 2 are position isomers. Molecule number 2 and 3 are chain isomers. Molecule number 3 and 4 are position isomers.
Additional Information Enantiomers are non-superimposable mirror images stereoisomers. Mixtures of enantiomers in equal amounts are known as racemic mixture or racemate. Racemic mixture is optically inactive due to external compensation. Enantiomers are having two types of absolute configurations named as R-configuration and S-configuration. Mirror image of R-isomer is always S-isomer and vice versa. R-isomer and S-isomer are non-superimposable mirror images of each other. R-isomer is an enantiomer of a S-isomer and vice versa.
Note: Structural formula of C4H10O are drawn by changing connectivity of atoms. Different Connectivity of carbon atoms and functional groups will give different constitutional isomers. Butan-2-ol molecules have a chiral carbon atom and it is optically active, so it shows optical isomerism.
