Question
Question: Write the name and structure of a saturated compound in which the carbon atoms are arranged in a rin...
Write the name and structure of a saturated compound in which the carbon atoms are arranged in a ring. Give the number of single bonds present in this compound.
Solution
Saturated compounds contain all the single bonds between carbon-carbon atoms. The structure in which all the carbon atoms are joined in a ring is called cycloalkanes. For example, three carbon atoms joined in a ring with a single bond called cyclopropane and it contains 9 single bonds (each carbon atom joined to two hydrogen atoms with a sigma bond).
Complete step by step solution:
In organic chemistry, a saturated compound is the one which contains all the single bonds in between carbon-carbon atoms. The structures in which the carbon atoms are arranged in a ring are called cycloalkanes. They have a general formula as CnH2n.
There can be many saturated cycloalkanes. For example, cyclopropane, cyclobutane, cyclopentane etc.
A brief example of a saturated cycloalkane :
Let us discuss cyclohexane and the structure of cyclohexane is:
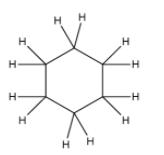
In the above structure, it is quite clear that six carbon atoms are arranged in a ring and each carbon atom is joined to two hydrogen atoms to complete its octet (carbon forms four single bonds to complete its octet). All the six carbon atoms are joined with a single bond that’s why the compound is saturated. Thus, cyclohexane contains 18 single bonds ( 6 carbon-carbon bonds and 12 carbon-hydrogen bonds).
Note: Simplest saturated cycloalkane is cyclopropane with three carbon atoms joined in a ring. A famous German organic chemist, A. Baeyer suggested that cyclobutane and cyclopropane are less stable than cyclohexane because the smaller rings are more strained. Angle strain can be seen in cyclobutane and cyclopropane which makes them less stable than cyclohexane and cyclopentane, which have a much lower ring strain.
