Question
Question: Write the monomers used for getting the following polymers. i) Polyvinyl chloride ii) Teflon ...
Write the monomers used for getting the following polymers.
i) Polyvinyl chloride
ii) Teflon
iii) Bakelite
Solution
Hint: In this question we have to tell the monomeric unit of the given polymers. The monomer unit is the unit used to form a polymer, or we can say the smallest unit of a polymer. Some monomer units can be identified from the name of a polymer itself.
Complete step by step solution:
Now, first we will define the polymers. It is formed by a combination of the same, or different monomer units; composing a large molecule.
Let us discuss the given polymer, and identify their monomer units one by one.
The first (i) we have is polyvinyl chloride. Now in this case from the name we can say that it is composed of vinyl chloride. As we know poly means many.
So, we can say that the vinyl chloride is the monomer unit of polyvinyl chloride. Its chemical formula is CH2=CHCl. We can represent the reaction as:
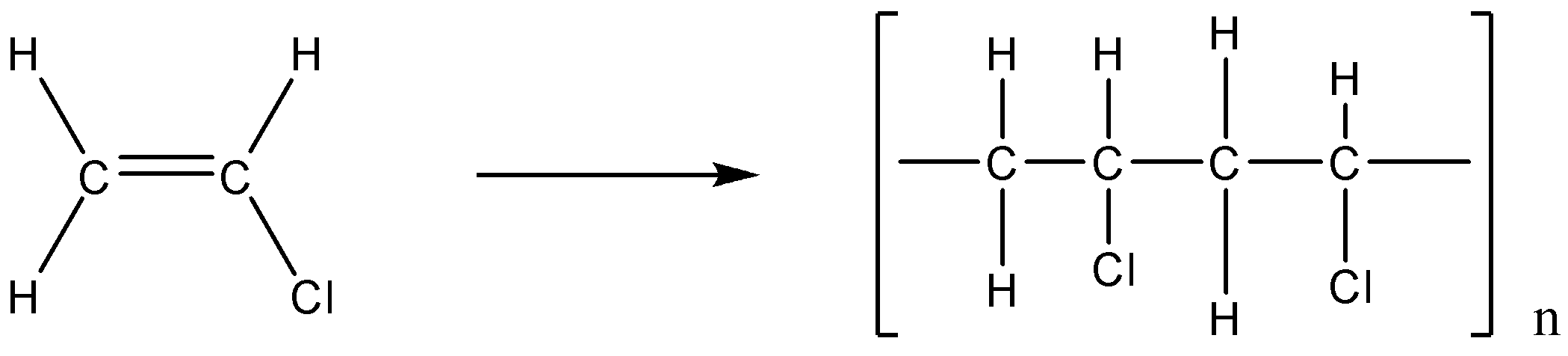
The second (ii) we have is Teflon; it is also known as polytetrafluoroethylene.
So, here we can say that the Teflon is composed of monomer units of tetrafluoroethylene. Its chemical formula is CF2=CF2.
The reaction can be written as:

The third (iii) we have is Bakelite. It is an example of a thermosetting polymer, and is not a homopolymer.
If we talk about the composition of Bakelite, it is composed of two monomer units i.e. phenol, and formaldehyde.
So, its chemical formula is HCHO(formaldehyde) + C6H5OH(Phenol).
We can represent the formation of bakelite as follows:
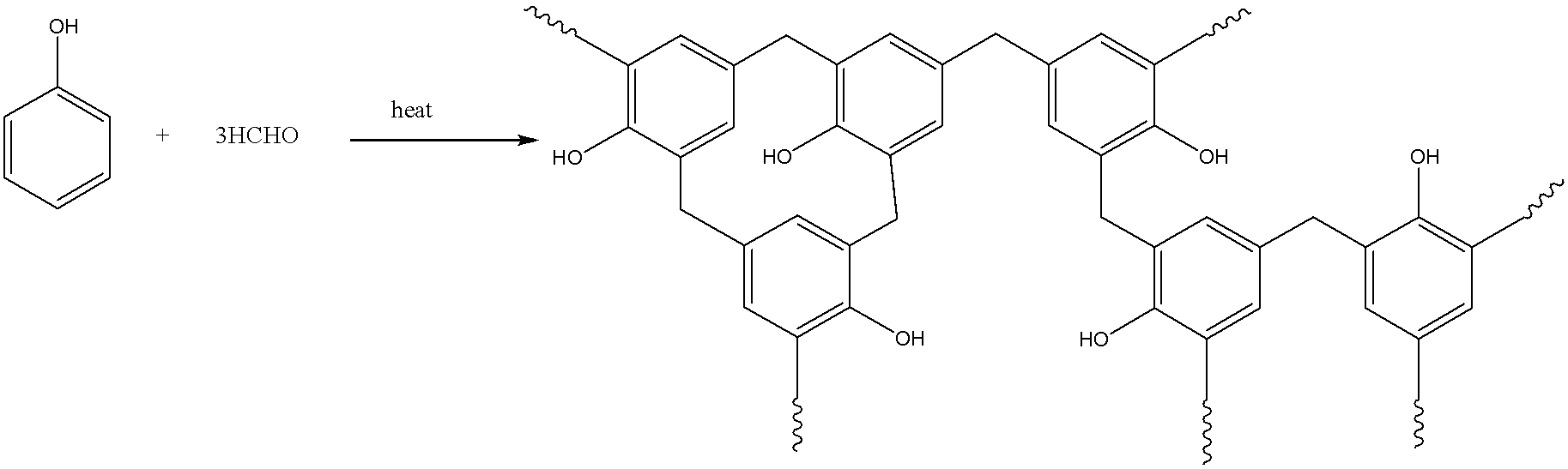
In the last we can conclude that first, and second are the homopolymers composed of one monomer unit i.e. vinyl chloride, and tetrafluoroethylene respectively. The third part is not a homopolymer composing of phenol, and formaldehyde.
Note: Don’t get confused while determining the monomer units of polymers. If you know their other names, or the chemical representation, then the unit can be identified easily.
