Question
Question: Write the mechanism of the reaction of HI with methoxy benzene....
Write the mechanism of the reaction of HI with methoxy benzene.
Solution
The organic compound anisole, also known as methoxy benzene, has the formula. It's a colourless liquid with an anise seed aroma, and many of its derivatives can be used in natural and synthetic fragrances. The compound is mostly synthesised and serves as a precursor to other synthetic compounds.
Complete answer:
Methoxy benzene forms phenol and iodomethane as it reacts with hydroiodic acid HI.
We can represent this reaction as,
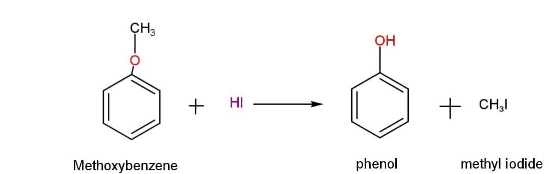
We know that methoxy benzene is alkyl aryl ether. So, methoxy benzene has two bonds that are joined by an oxygen atom. The oxygen-methyl group (O-alkyl bond) is one, and the oxygen-aryl group is the other (O-Aryl bond). Because of the partial double bond character of the Oxygen-Aryl bond, it is the most stable of the three.
That is, oxygen-methyl bond is cleaved in this reaction, resulting in methyl iodide and phenol.
The reaction between HI and methoxy benzene occurs in two stages and they are;
Stage 1: By trapping H+ from HI, the oxygen in ether is protonated (added a proton), hence forming a protonated ether molecule known as oxonium ion.
Stage 2: Since Iodide ion (I-) is a strong nucleophile, the SN2 reaction occurs in this step. To form methyl iodide and phenol, the I- ion preferentially targets the protonated ether molecule's least substituted carbon (in this case, the methyl group).
Note:
We should also remember that phenol is an aromatic organic compound with the molecular formula. The chemical compound with the formula CH3I is iodomethane, also known as methyl iodide and commonly abbreviated "MeI."
