Question
Question: Write the mechanism of the following reaction: \(C{{H}_{3}}C{{H}_{2}}OH\xrightarrow{HBr}C{{H}_{3}}...
Write the mechanism of the following reaction:
CH3CH2OHHBrCH3CH2Br+H2O
Solution
in the first part we are given a reaction in which ethyl alcohol reacts with hydrogen bromide. In the reaction, an electron rich compound replaces the leaving group. To solve the second part we must know that in the Reimer-Tiemann reaction phenol is converted to an ortho hydroxy Benzaldehyde.
Complete step by step solution:
We are given the reaction as follows: CH3CH2OHHBrCH3CH2Br+H2O
In the given reaction, ethanol i.e. alcohol reacts with hydrogen bromide. The product formed is ethyl hydrogen bromide which is an alkyl halide along with water which is the by-product of the reaction. In the reaction, an electron rich compound i.e. the bromine atom replaces the hydroxyl leaving group. The reaction in which an electron rich compound replaces the leaving group is known as nucleophilic substitution reaction.
The lone pair on oxygen attacks the HBr molecule forming a hydronium ion.
Formation of carbocation.
Attack of Nucleophile Br−
The mechanism of the reaction is as follows:
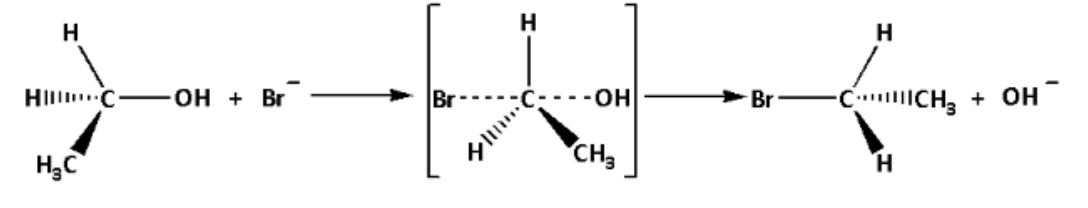
In the reaction mechanism, we can see that the simultaneous breaking and making of the bond occurs. The configuration is inverted. Thus, the mechanism of the reaction is i.e. nucleophilic substitution bimolecular mechanism.
Additional Information:
In the Reimer-Tiemann reaction, phenol is converted to an ortho hydroxybenzaldehyde. It is a type of substitution reaction. In the Reimer-Tiemann reaction, phenol is treated with chloroform in the presence of sodium hydroxide. Thus, an aldehyde group is introduced at the ortho-position of the benzene ring. This leads to the formation of ortho-hydroxybenzaldehyde.
Note: The reaction is a nucleophilic substitution reaction in which bond breaking and bond formation occurs simultaneously. The reaction mechanism requires the attack of nucleophiles from the back side of the carbon atom. Thus, the product obtained has the configuration opposite to that of the reactant. This is known as inversion of configuration
