Question
Question: Write the mechanism of nucleophilic substitution, when p-nitro chlorobenzene is treated with caustic...
Write the mechanism of nucleophilic substitution, when p-nitro chlorobenzene is treated with caustic soda.
Solution
Reactions involving replacement of halo group of aryl halide with some other substituent is known as nucleophilic aromatic substitution reaction. Aryl halides are less reactive than alkyl halides towards nucleophilic substitution reaction.
Complete step by step solution:
We know that aryl halides are resonance stabilized due to delocalization of electrons, so that C−Xbond acquires some double bond character which makes its cleavage difficult.
Thus aryl halides undergo nucleophilic substitution reaction at high temperature and pressure only.
Now let us look into the mechanism of the reaction
We have caustic soda which is the NaOH, the OH−of caustic soda attacks the chlorine part in the benzene
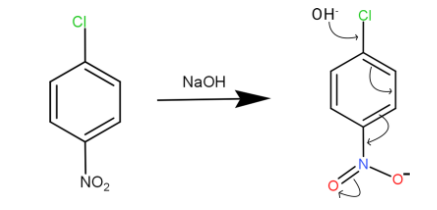
And when this happens due to resonating structure and the electron withdrawing group shift the electrons to accommodate the OH−. We can see the bond shift marked by the arrows.
This compound is very unstable and it again goes to its resonating structure
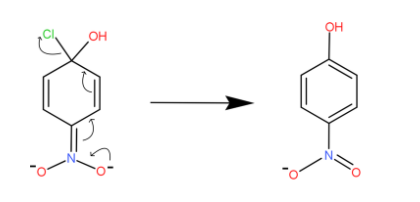
The electrons on the one side of nitro pass into its nitrogen bond and it circulates throughout the structure. We can see the movement of electrons or bonds by arrow marks. At last the bond on the chlorine atom shifts towards it and it gets detached.
Finally, we have the chlorine atom detached and hydroxide gets attached to that position. This is how the halo group is replaced in aryl halides.
Note: Here apart from chlorine attached to the benzene group we have an electron withdrawing group −NO2 at the para position. Presence of a strong electron withdrawing group at ortho or para position enhances the reactivity of aryl halides towards nucleophilic substitution.
We can also say that with the presence of an electron withdrawing group the reaction can occur in less temperature and pressure.
