Question
Question: Write the lewis structure of Oxygen (\({{O}_{2}}\)) and ethyne (\({{C}_{2}}{{H}_{2}}\)) molecules....
Write the lewis structure of Oxygen (O2) and ethyne (C2H2) molecules.
Solution
A Lewis Structure is a very simplified representation of the valence shell electrons in a molecule. It is used to show how the electrons are arranged around individual atoms in a molecule. Electrons are shown as "dots" or for bonding electrons as a line between the two atoms. The goal is to obtain the "best" electron configuration, i.e. the octet rule and formal charges need to be satisfied.
Complete step by step answer:
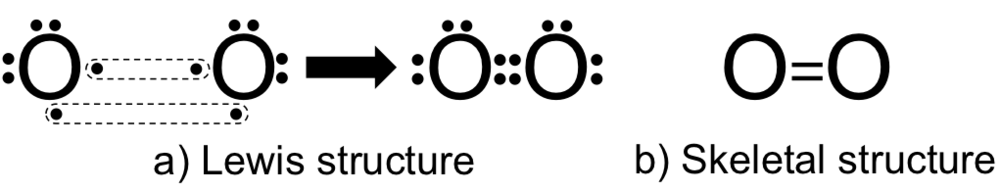
Electronic configuration of oxygen atom: 1s22s22p4
Oxygen atom has six electrons in its valence shell and to fulfill its octet it needs 2 more electrons.
- Now here for these two electrons it is combined with another oxygen atom and both atoms will 2 electrons a piece and make a double bond between them, so four electrons will be shared.
Lewis structure is shown below:
Lewis structure of ethyne:
- Electronic configuration of Carbon atom: 1s22s22p2
To show tetravalency one 2s electron will move into empty 2p orbital
New electronic configuration of Carbon atom: 1s22s12p3
To complete its octet each carbon needs 4 electrons.
Electronic configuration of hydrogen: 1s1
To have a configuration like He, hydrogen needs one electron.
- So here both carbons can combine with one hydrogen but that will only give each carbon one electron and for the remaining three electrons, these carbons will combine together and share 3 electrons a piece and a triple bond will form and now each has 8 electrons, where 1 electron it is getting from 1 hydrogen and three electrons it is getting from other carbon atom.
Its lewis structure is shown below:
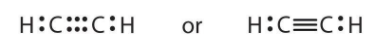
Additional Information : Total number of bonds between two atoms will always be equal to half of the number of total electrons shared. As in above case 6 electrons were shared that’s why both carbon atoms have triple bonds between them.
Note: Ethyne is a colorless gas with a characteristic smell. It has a melting point −810C. It has a boiling point −840C. It is a poisonous gas and liquid ethyne is explosive. It is lighter than air and significantly soluble in water. It has linear structure.
